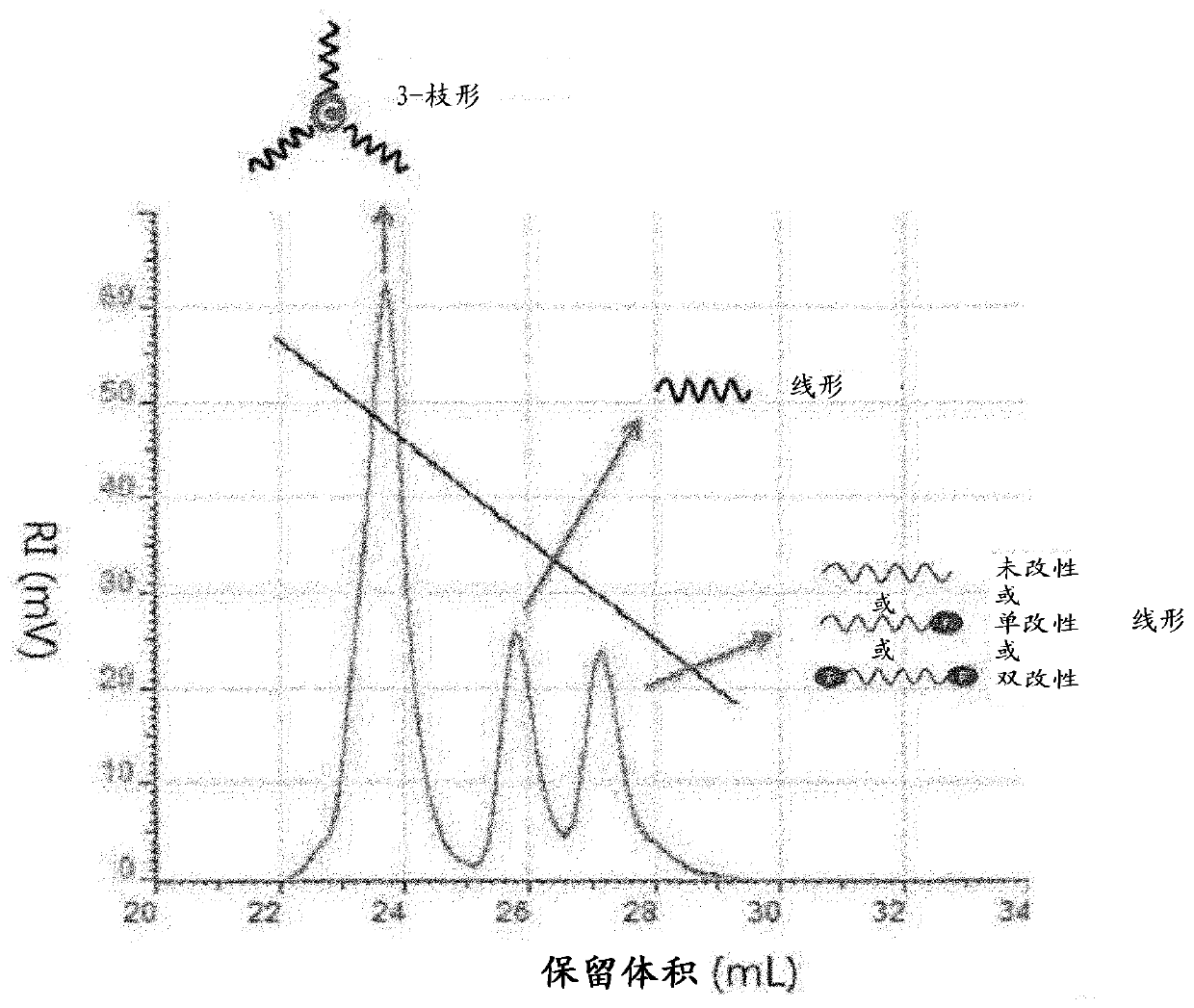
Method for producing modified conjugated diene polymer and rubber composition using polymer produced by the method
A manufacturing method and technology of copolymers, which are applied in transportation and packaging, special tires, tire parts, etc., can solve problems such as difficult to achieve branched structure, difficult to adjust polymer microstructure, and difficult to adjust content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
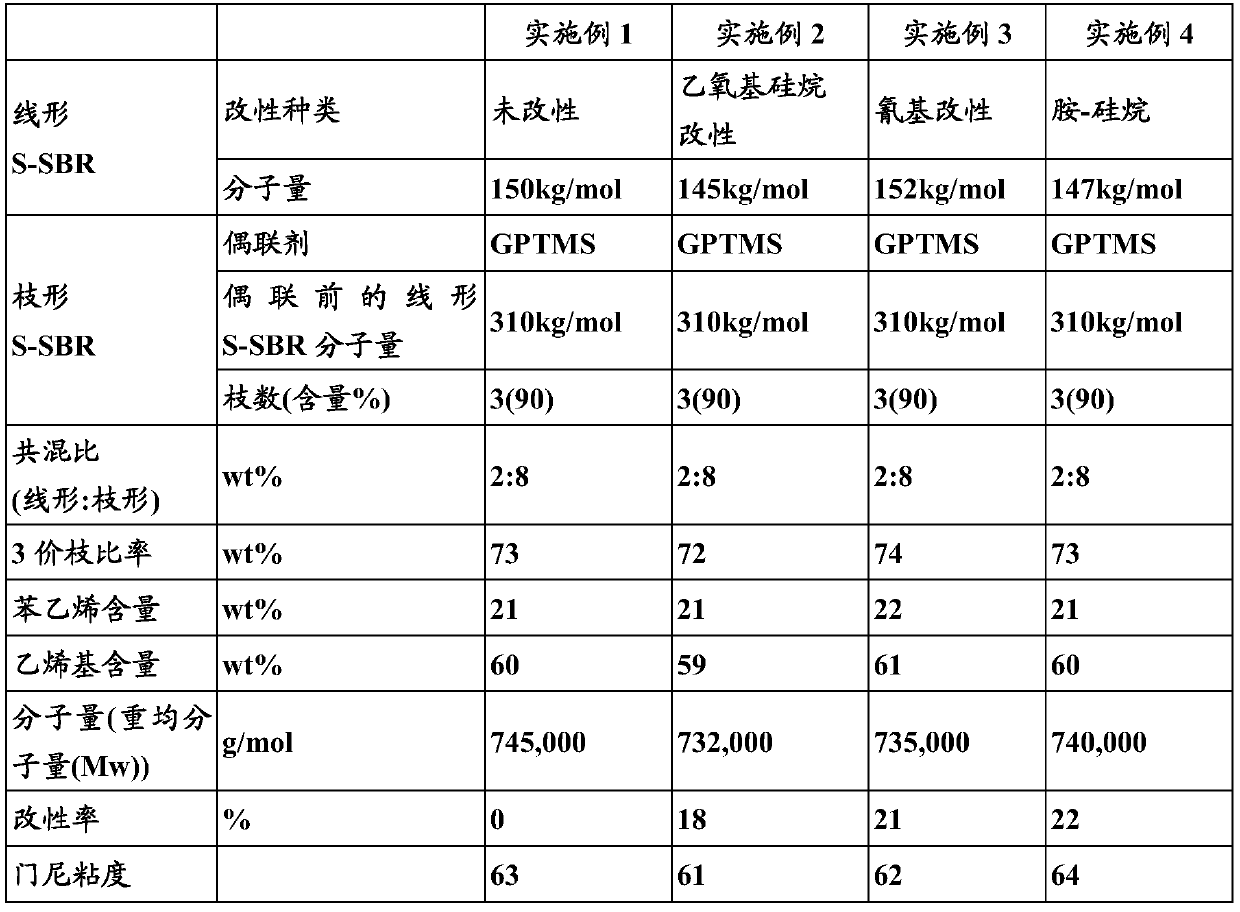
Embodiment 1
[0066] 1) Manufacture of linear SBR
[0067] Put 170g of styrene, 630g of 1,3-butadiene, and 4150g of hexane into a 10L autoclave reactor, add 10ml of tetramethylethylenediamine (TMEDA), and raise the temperature of the reactor while rotating it with a stirrer to 50°C. Butyllithium (BuLi) was placed here, and a linear SBR having a weight average molecular weight (Mw) of about 150,000 g / mol was polymerized through a polymerization reaction. After 10 minutes had elapsed since the reaction temperature reached the highest temperature, 50 g of butadiene was charged, and the terminal end of the polymer was replaced with a butadiene active anion over 5 minutes. Thereafter, ethanol was added as a reaction terminator to terminate the polymerization, and 0.2 wt% of I-1076 was added as an antioxidant to the polymer to prepare a linear SBR solution.
[0068] 2) Manufacture of Dendritic SBR
[0069]Put 170g of styrene, 630g of 1,3-butadiene, and 4150g of hexane into a 10L autoclave reac...
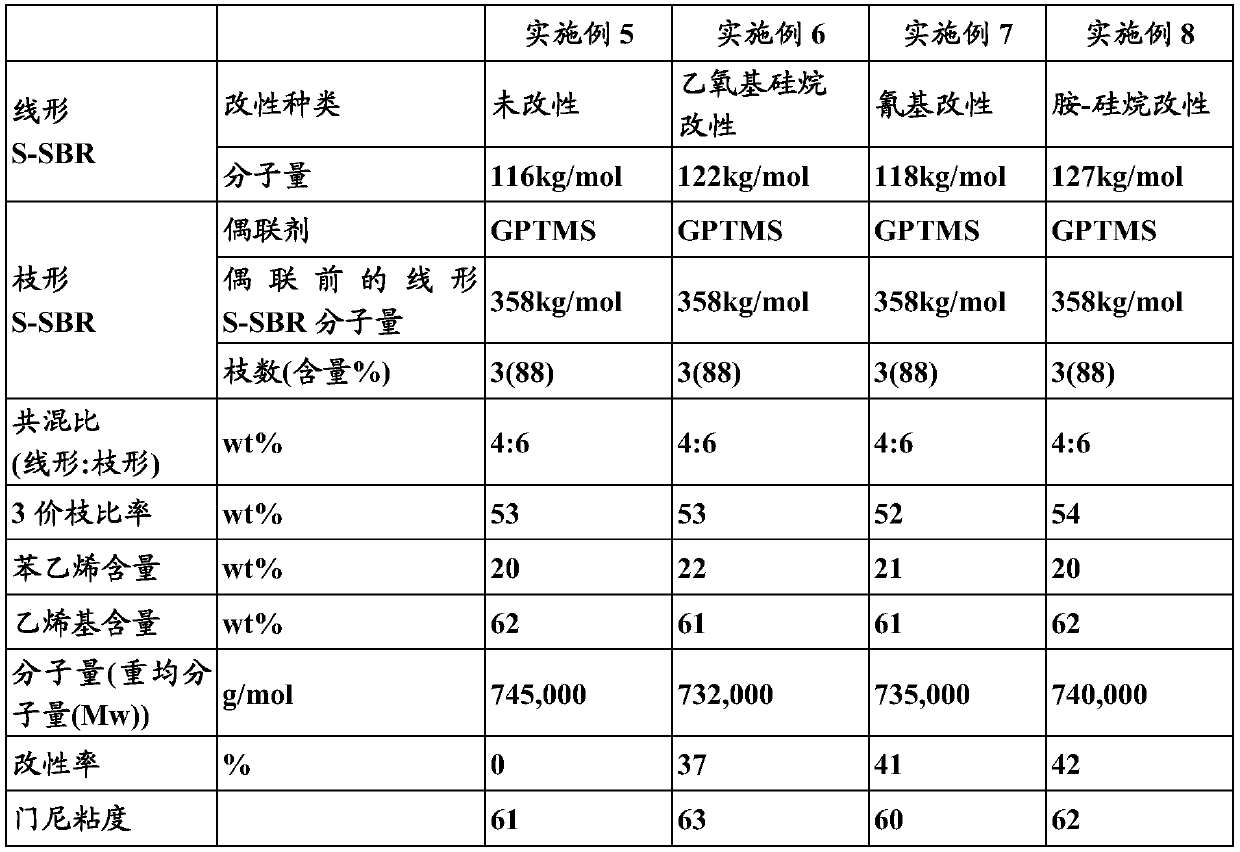
Embodiment 2
[0073] 1) Manufacture of linear SBR
[0074] Put 170g of styrene, 630g of 1,3-butadiene, and 4150g of hexane into a 10L autoclave reactor, add 10ml of tetramethylethylenediamine (TMEDA), and raise the temperature of the reactor while rotating it with a stirrer to 50°C. Butyllithium (BuLi) was placed here, and a linear SBR having a weight average molecular weight (Mw) of about 150,000 g / mol was polymerized through a polymerization reaction. After 10 minutes had elapsed since the reaction temperature reached the highest temperature, 50 g of butadiene was charged, and the terminal end of the polymer was replaced with a butadiene active anion over 5 minutes. Here, fluorobutyllithium (BuLi) in which a modifier diethoxydimethylsilane (diethoxydimethylsilane) was charged at a molar ratio of 1.2 moles was charged, and reacted for 10 minutes. Thereafter, ethanol was added as a reaction terminator to stop the polymerization, and 0.2 wt% of I-1076 was added as an antioxidant to the pol...
Embodiment 3
[0080] 1) Manufacture of linear SBR
[0081] Put 170g of styrene, 630g of 1,3-butadiene, and 4150g of hexane into a 10L autoclave reactor, add 10ml of tetramethylethylenediamine (TMEDA), and raise the temperature of the reactor while rotating it with a stirrer to 50°C. Butyllithium (BuLi) was placed here, and a linear SBR having a weight average molecular weight (Mw) of about 150,000 g / mol was polymerized through a polymerization reaction. After 10 minutes had elapsed since the reaction temperature reached the highest temperature, 50 g of butadiene was charged, and the terminal end of the polymer was replaced with a butadiene active anion over 5 minutes. Here, butyllithium (BuLi) in which a modifier (3-cyanopropyl)dimethylchlorosilane ((3-Cyanopropyl)dimethylchlorosilane) was charged at a molar ratio of 1.2 moles was added and reacted for 10 minutes. Thereafter, ethanol was added as a reaction terminator to terminate the polymerization, and 0.2 wt % of terminator I-1076 was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com