Combined preparation method of dalteparin sodium and nadroparin calcium
A technology of nadroparin calcium and dalteparin sodium, applied in the field of chemical pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1, weigh 2 kg of heparin sodium, dissolve it, adjust the pH to 2.0-3.5, then add sodium nitrite, stir and react for 6 hours to obtain a degradation feed solution, in which the weight-average molecular weight of low-molecular-weight heparin is 2818.
[0017] Adjust the pH of the degradation feed solution to neutral, add sodium borohydride, stir and react at 10°C-30°C for 6 hours, adjust the pH of the feed solution to 4.0-4.5, and stir for 1 hour to obtain a reduction feed solution.
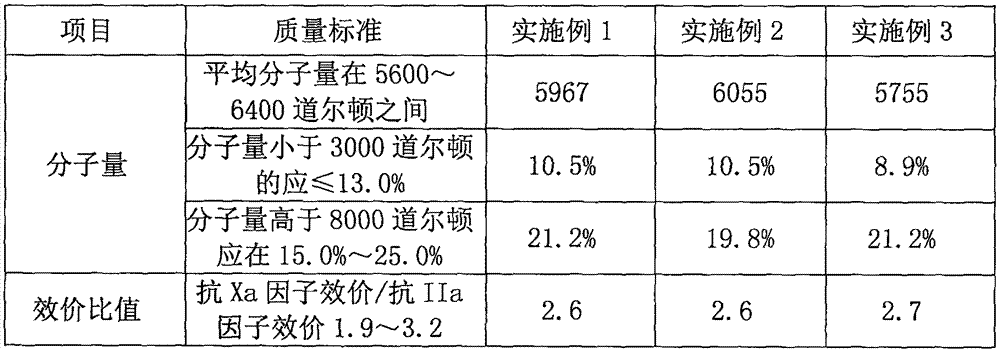
[0018] Add the reducing material solution to ethanol for fractional precipitation, and control the degree of ethanol to 35°. Collect the precipitate, dissolve it with 5 times the weight of water, add 3% sodium chloride, and then grade the precipitate with ethanol. The degree of ethanol is controlled at 35°, collect the precipitate, and detect the weight average molecular weight of low molecular weight heparin in the precipitate to be 5967. After the precipitation was dried, daltepari...
Embodiment 2
[0020] Example 2, weigh 2 kg of heparin sodium, dissolve it, adjust the pH to 2.0-3.5, then add sodium nitrite, stir and react for 5 hours to obtain a degradation feed solution, in which the weight-average molecular weight of low-molecular-weight heparin is 3591.
[0021] Adjust the pH of the degradation feed solution to neutral, add sodium borohydride, stir and react at 10°C-30°C for 6 hours, adjust the pH of the feed solution to 4.0-4.5, and stir for 1 hour to obtain a reduction feed solution.
[0022] Add the reduced raw material solution to ethanol for fractional precipitation, control the degree of ethanol to 45°, collect the precipitate and detect the weight average molecular weight of low molecular weight heparin in the precipitate to be 6055. After the precipitation was dried, dalteparin sodium was obtained, with a weight of 0.69 kg and a yield of 34.5%.
[0023] Add 2 times the volume of ethanol to the upper layer feed solution in the ethanol fractionation precipitati...
Embodiment 3
[0024] Example 3, weigh 2 kg of heparin sodium, dissolve it, adjust the pH to 2.0-3.5, then add sodium nitrite, stir and react for 6 hours to obtain a degradation feed solution, in which the weight-average molecular weight of low molecular weight heparin is 3346.
[0025] Adjust the pH of the degradation feed solution to neutral, add sodium borohydride, stir and react at 10°C-30°C for 6 hours, adjust the pH of the feed solution to 4.0-4.5, and stir for 1 hour to obtain a reduction feed solution.
[0026] Add the reduced raw material solution to ethanol for fractional precipitation, control the degree of ethanol to 40°, collect the precipitate and detect the weight average molecular weight of low molecular weight heparin in the precipitate to be 5755. After the precipitation was dried, dalteparin sodium was obtained, with a weight of 0.60 kg and a yield of 30%.
[0027] Add 2 times the volume of ethanol to the upper layer feed solution in the ethanol fractionation precipitation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com