Method for preparing benzothiazole quinazoline derivatives through catalysis
A technology for preparing benzothiazoquinazoline and catalysis, which is applied in the fields of chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., and can solve the problems of less recyclable times, large amount of catalyst usage, and complicated operation process and other problems, to achieve the effect of more recycling times, shorter reaction time and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] For the preparation method of the bissulfonate acidic ionic liquid catalyst, see the relevant literature (Novel multiple-acidic ionic liquids: catalysts for environmentally friendly benign synthesis of trans-β-nitrostyrenes under solvent-free conditions, Industrial & Engineering Chemistry Research, 2014, 53: 547~ 552).
[0035] A kind of method for catalyzing the preparation of benzothiazole quinazoline derivatives provided by the present invention, the specific technical process is shown in figure 1 , the chemical reaction formula of this reaction is:
[0036]
[0037] Wherein the molar ratio of 2-aminobenzothiazole derivative (1), α-tetralone (2) and aldehyde (3) in the reaction is 1:1:(1~1.1), and the bissulfonate acidic ionic liquid catalyst The molar dosage is 5~8% of the molar amount of the 2-aminobenzothiazole derivative used, and the volume of the reaction solvent water in millimoles is 5~8% of the molar amount of the 2-aminobenzothiazole derivative in milli...
Embodiment 1
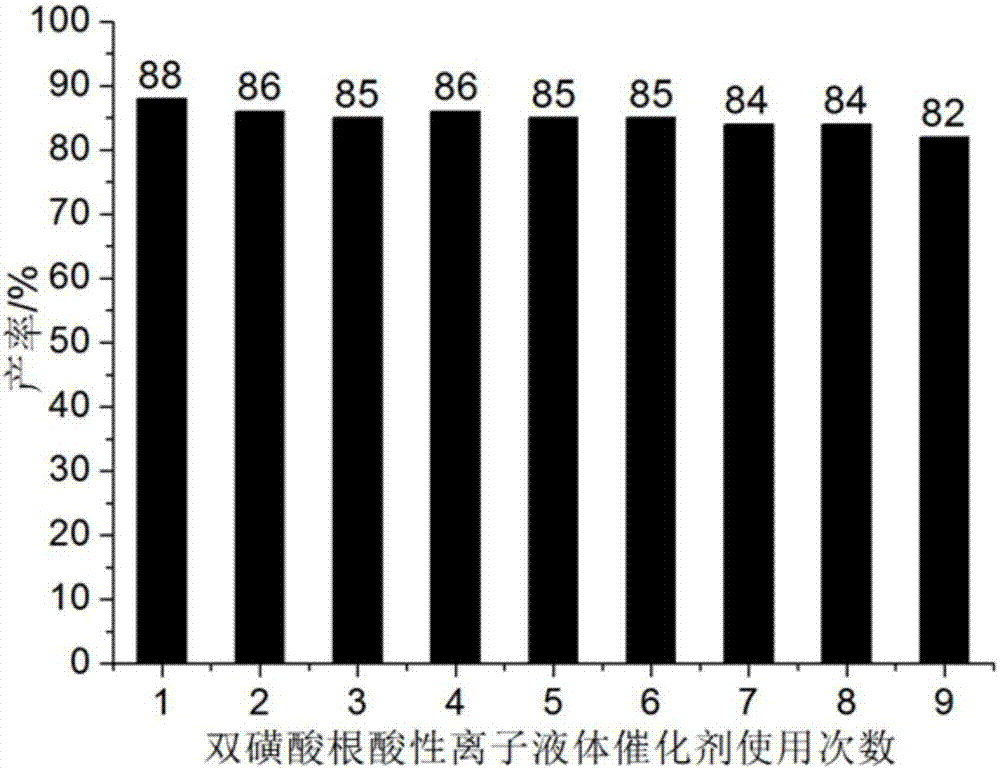
[0044] Add 1mmol of 4-chloro-2-aminobenzothiazole, 1mmol of α-tetralone, 1mmol of furfuraldehyde and 0.06mmol of bissulfonate acidic ionic liquid to 25ml of single port with stirring bar and condenser tube filled with 5ml of water in the bottle. Reaction at 68°C for 29min, followed by TLC (thin plate chromatography), cooled to room temperature after the reaction, crushed the precipitated solid, let stand, filtered with suction, washed the filter residue with ethanol (3ml×3), and dried in vacuo to obtain 9- Chloro-7-(furan-2-yl)-5,6-dihydro-7H-benzo[h]benzothiazo[2,3-b]quinazoline, the yield is 88%, make up 5ml with water The final filtrate was directly added with 4-chloro-2-aminobenzothiazole, α-tetralone and furanformaldehyde and reused, and the collected ethanol washing liquid was used for the next washing.
[0045] The performance parameters of 9-chloro-7-(furan-2-yl)-5,6-dihydro-7H-benzo[h]benzothiazo[2,3-b]quinazoline obtained in this example are: m.p.219~221℃; IR(KBr):...
Embodiment 2~5
[0051] The preparation methods of Examples 2-5 are basically the same as in Example 1, the difference mainly lies in the different reaction temperatures. The specific reaction temperatures and the yields of the products obtained in each embodiment are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com