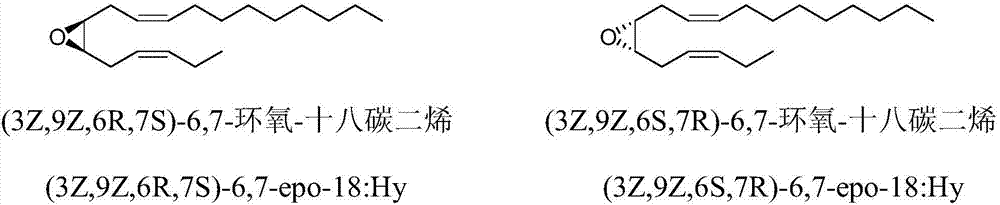
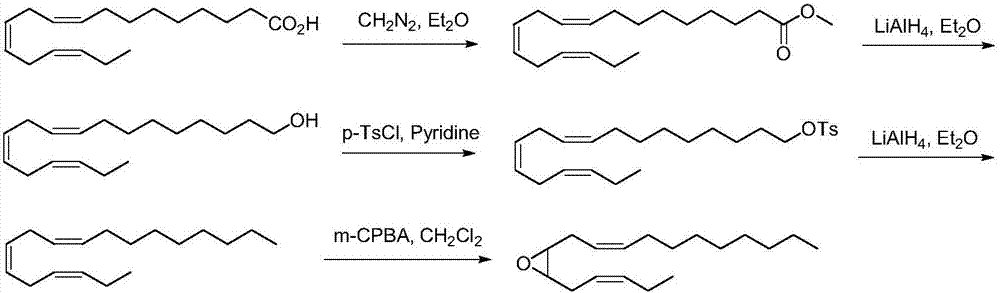
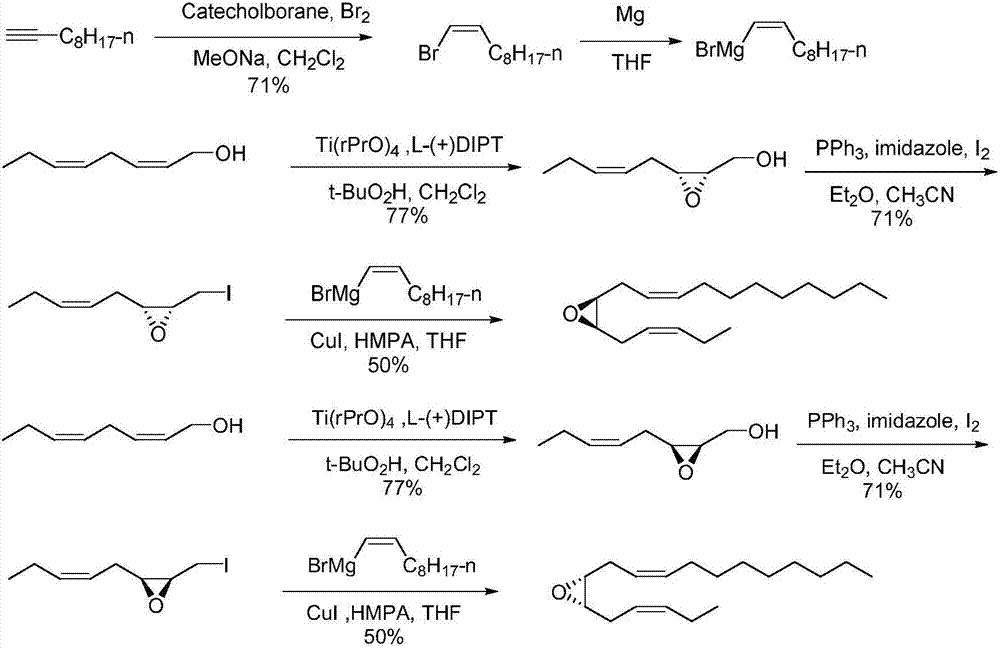
Synthetic method of tea geometrid tea geometrid
A synthesis method and pheromone technology, applied in organic chemistry methods, organic chemistry and other directions, can solve problems such as harsh reaction conditions, and achieve the effect of simple operation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Dissolve AD-mix-alpha (20g) in t-BuOH:H 2 O=1:1 (140mL) solution, cooled to 0°C, added (2E)-2-alkenyl-5-ynyl-1-octanol (1.8g, 14.5mmol) and MeSO 4 NH 2 (1.4g, 14.5mmol), reacted at 0°C for 3 days. After the reaction, NaHSO was added at 0°C 3 (18g) quenched, suction filtered, and the filtrate was extracted with ethyl acetate, washed with saturated brine, and the organic phase was collected, washed with Na 2 SO 4 dry. Spin-dried and passed through the column to obtain (2R,3R)-5-alkynyl-1,2,3-trioctanol (1.9 g, yield 85%) as a white solid. 1 H-NMR (400MHz, CDCl 3 ): δ1.12(t, J=7.5Hz, 3H), 2.17(q, J=7.5Hz, 2H), 2.47(t, J=16.8Hz, 2H), 3.75(m, 4H), 4.01(s ,1H); 13 C-NMR (400MHz, CDCl 3 ): δ83.4, 76.3, 76.0, 75.7, 74.0, 71.7, 70.0, 63.5, 23.0, 13.0, 11.3.
[0066] Step 2: Preparation of (6R,7R)-7,8-epoxy-6-p-toluenesulfonate-3-octyne
Embodiment 2
[0067] Example 2: Add NaH (2.87g, 72mmol) to THF (240mL), add (2R,3R)-5-alkynyl-1,2,3-trioctanol (3.78g, 24mmol) at 0°C , Tos-Im (19.98 g, 59 mmol). The reaction was performed at 0°C and detected by TLC. After the reaction is complete, add H at 0°C 2 O quenched, extracted with ethyl acetate, washed with saturated brine, collected the organic phase, and washed with Na 2 SO 4 dry. Spin-dried and passed through the column to obtain (6R,7R)-7,8-epoxy-6-p-toluenesulfonate-3-octyne (4 g, yield 41%) as a colorless transparent liquid. 1 H-NMR (300MHz, CDCl 3 ): δ1.07(t, J=7.5Hz, 3H), 2.10(qt, J=7.5, 2.4Hz, 2H), 2.45(s, 3H), 2.61-2.65(m, 2H), 2.73(dd, J=4.8,2.5Hz,2H),2.83(m,1H),3.20(m,1H),4.32(dd,J=13.4,6.3Hz,1H),7.34(d,J=8.0Hz,2H), 7.82(d, J=8.3Hz, 2H); 13 C-NMR (400MHz, CDCl 3 ): δ143.8, 132.8, 128.9, 128.7, 127.0, 126.9, 79.6, 71.5, 66.9, 51.2, 44.3, 21.8, 20.6, 12.8, 11.2.
[0068] Step 3: Preparation of (6S,7S)-3,9-diyne-6-p-toluenesulfonate-7-octadecyl alcohol
Embodiment 3
[0069] Embodiment 3: Take 1-decyne (580mg, 4.2mmol) in THF, N 2 For protection, add n-BuLi (1.2mL, 2.8mmol, 2.5M in hexane) at room temperature, react at room temperature for 15min, cool down to -78°C, add (7R,8R)-7,8-epoxy-6-p-toluene Sulfonate-3-octyne (569mg, 2.4mmol), BF 3 ·Et 2 O (0.264mL), reacted overnight. After the reaction was completed, it was quenched by adding saturated ammonium chloride at -78°C, raised to room temperature and stirred for 30 minutes, spin-dried THF, extracted with ethyl acetate, washed with saturated brine, collected the organic phase, and washed with Na 2 SO 4 dry. Spin-dried and passed through the column to obtain a slightly oily liquid (398 mg, yield 51%). 1 H-NMR (300MHz, CDCl 3 ): δ0.88(t, J=6.7Hz, 3H), 1.08(t, J=7.5Hz, 3H), 1.27(m, 10H), 1.42-1.50(m, 2H), 2.05-2.17(m, 4H),2.25-2.40(m,2H),2.45(s,3H),2.50-2.59(m,1H),2.74(qt,J=8.1,2.4Hz,1H),3.98-4.05(m,1H) ,4.61-4.67(m,1H),7.34(d,J=8.0Hz,2H),7.82(d,J=8.3Hz,2H); 13 C-NMR (300MHz, CDCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com