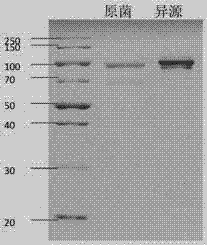
Collagenase producing strain as well as collagenase gene sequence and application thereof
A technology of collagenase and bacterial strains, which is applied in application, genetic engineering, plant gene improvement, etc., can solve the problems of difficult to fully utilize, difficult digestion and absorption of collagen macromolecules, and achieve a wide range of pH applications, strong temperature tolerance, The effect of high enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 strain Col15 Screening, identification and enzyme-producing properties of
[0029] 1. Screening of collagenase-producing strain Col15
[0030] The applicant used soil samples collected near the Tianjin Meat Factory as the matrix, mixed them with sterile water and egg whites in a certain proportion, then added small pieces of pig skin and fish skin, and enriched at 25-30 °C Cultured; then diluted and spread to contain 2 % gelatin The LB flat plate was re-screened by the transparent circle of acidic mercury reagent, and then the strains of the primary screening were passed through the shake flask, and the enzyme activity was measured respectively, and finally the bacterial strain of the present invention was obtained.
[0031]2. Identification of collagenase-producing strain Col15
[0032] 1. Morphological characteristics of collagenase-producing strain Col15
[0033] The colony of the collagenase-producing strain Col15 in the present invention is w...
Embodiment 2
[0047] Example 2 strain Col15 Fermentation optimization research
[0048] The fermentation medium and fermentation conditions for the production of collagenase by strain Col15 were optimized by single factor experiments. Add 50 mL of fermentation medium to a 250 mL Erlenmeyer flask, sterilize with high pressure steam at 121 °C for 30 min, and sterilize the monosaccharides in the medium separately, and then add them to the medium after sterilization and cooling. All experiments were designed in triplicate, and the optimization results of the previous step were used in subsequent experiments.
[0049] (1) Determination of the optimum carbon source for the medium
[0050] When preparing the culture medium, 2% of different carbon sources were added, and other components remained unchanged. The enzyme activity was determined after 54 hours of fermentation in shake flasks. The results showed that the optimum carbon source of Col15 strain was glucose, and other carbon sour...
Embodiment 3
[0059] Example 3 strain Col15 Fish skin degradation experiment
[0060] After the strain Col15 was revived and activated, it was fermented at 37 °C and 200 rpm for 54 h, centrifuged at 5500 rpm for 30 min, and the supernatant was collected to obtain the crude enzyme solution of the strain Col15. The culture medium of the uncultivated strain Col15 was used as a control.
[0061] Thaw cod skin with tap water, remove excess minced meat and fish scales, cut into 5 mm x 5 mm pieces, fully drain, weigh and divide into two equal parts, add the drained equal parts of fish skin to In the Erlenmeyer flask containing 50 mL of crude enzyme solution and control medium, react in a water bath shaker at 37 °C and 120 rpm for 48 h. With reference to the control, it can be clearly seen that the cod skin has degraded.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com