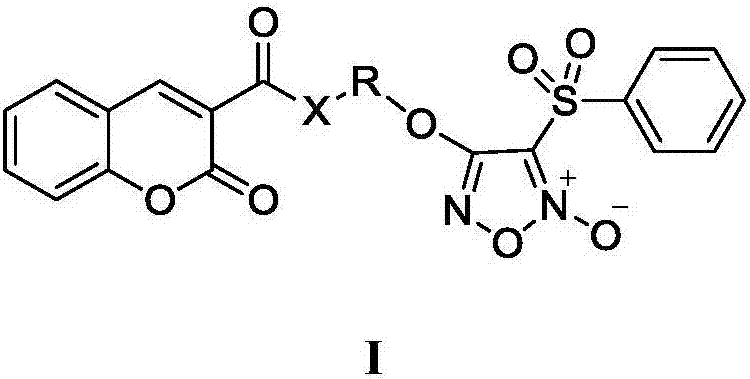
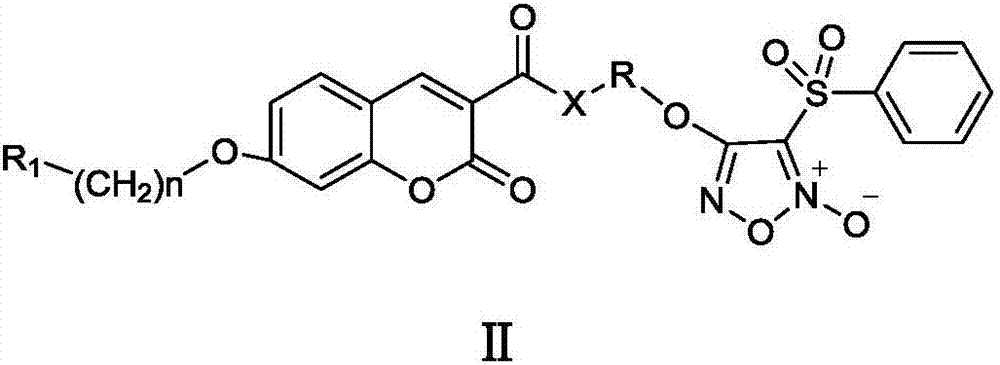
Nitric oxide donor type coumarin derivatives, preparation methods therefor and medicinal use of nitric oxide donor type coumarin derivatives
A technology of coumarin derivatives and body shape, which is applied in the fields of medicinal chemistry and pharmacotherapeutics, and can solve problems such as low bioavailability, limited clinical application, and unstable metabolism in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
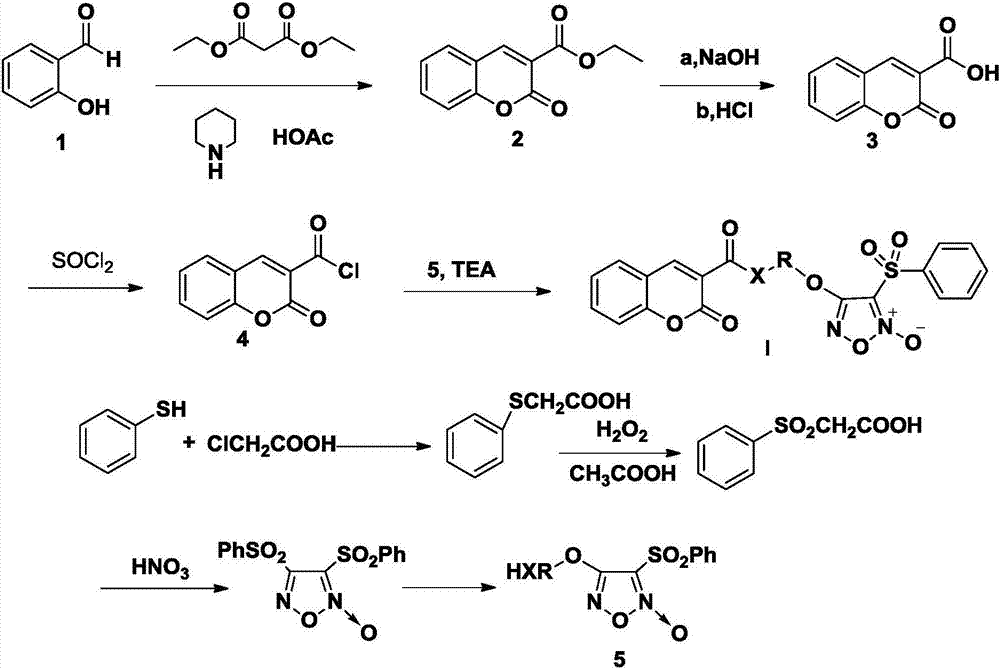
[0508] Synthesis of Coumarin-3-Carboxylic Acid (3)
[0509] In a dry 100mL round bottom flask, add 4.2mL (0.04mol) salicylaldehyde, 8.5mL (0.056mol) diethyl malonate, 25mL absolute ethanol, 2 drops of glacial acetic acid and 0.5mL hexahydropyridine, and heat Reflux for 2h. After cooling, the reactant was poured into water and crystallized in ice water. The crude ethyl coumarin-3-carboxylate was obtained by suction filtration, and 7.5 g of white crystals were obtained by recrystallization, with a yield of 85.4%, m.p.92.0-93.0°C.
[0510] In a 100mL round bottom flask, add 25mL of 95% ethanol and NaOH solution (8g of solid NaOH dissolved in 50mL of water), 6g (0.028mol) of ethyl coumarin-3-carboxylate, and heat to reflux for 15min. The reaction mixture was poured into 70 mL aqueous hydrochloric acid solution with stirring, and crystals were precipitated. Suction-filtered crude coumarin-3-carboxylic acid was recrystallized from 50% ethanol to obtain 5.2 g of white crystal coum...
Embodiment 2
[0520] Referring to the method of Example 1, coumarin-3-formyl chloride and 3,4-diphenylsulfonyl-1,2,5-oxadiazole-2-oxide were prepared.
[0521] Preparation of Aminoethanol Furazan (5)
[0522] Add NaH (142mg, 5.9mmol) and aminoethanol (0.2ml, 3.33mmol) into a 50ml round bottom flask, keep away from light, stir in an ice-salt bath, add furazan dissolved in THF, that is, 3,4-dibenzenesulfonate Acyl-1,2,5-oxadiazole-2-oxide (0.5g, 1.37mmol), keep the reaction on ice bath. TLC detected that the reaction was complete. Water was added to the reaction solution, and after extraction with EtOAc, the organic layer was washed with saturated NaCl, and TLC detected that the organic layer had a single spot. Anhydrous Na 2 SO 4 After drying, it was concentrated to dryness under reduced pressure and weighed 239 mg. Yield 62%.
[0523] Coumarin-3-carboxylic acid-ethanolamine furazan (I 6 )Synthesis
[0524] Dissolve ethanolamine furazan (285mg, 1.0mmol) and triethylamine (0.83ml, 6.0...
Embodiment 3
[0526] Referring to Example 1, 1,4-butynediol furoxan was synthesized.
[0527] Synthesis of 2,4-Dihydroxybenzaldehyde
[0528] Add DMF (19mL, 210mmol) and acetonitrile (100mL) into a 500mL three-necked flask, and stir well at room temperature. Phosphorus oxychloride (19 mL, 250 mmol) was slowly added dropwise in an ice bath, and the addition was completed after 1 h, and then stirred at room temperature for 1 h. Dissolve 1,3-benzenediol (21g, 190mmol) in acetonitrile, and slowly add it dropwise to the reaction solution, a white precipitate is formed. After the dropwise addition, the solution is stirred for 4 hours in an ice bath and 2 hours at room temperature. Filter with suction, wash the filter cake with ice acetone, and vacuum-dry the obtained solid at 50°C to constant weight. Transfer the solid to a 500mL round bottom flask, add 400mL of water, heat and stir at 50°C for 30min, then stir at room temperature for 2h, a pink needle-like solid precipitates. The crude produc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com