Carbon fluoride and preparation method and application thereof
A carbon fluoride, the technology of carbon fluoride, which is applied in electrical components, battery electrodes, non-aqueous electrolyte batteries, etc., can solve the problems of limited industrialization development, poor storage performance, unstable carbon fluoride material structure, etc. Large market application prospect, simple preparation method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (1) Put 10g of carbon powder into the fluorination equipment, the C content is 80%, the N content is 8%, the H content is 5%, and the O content is 7%;
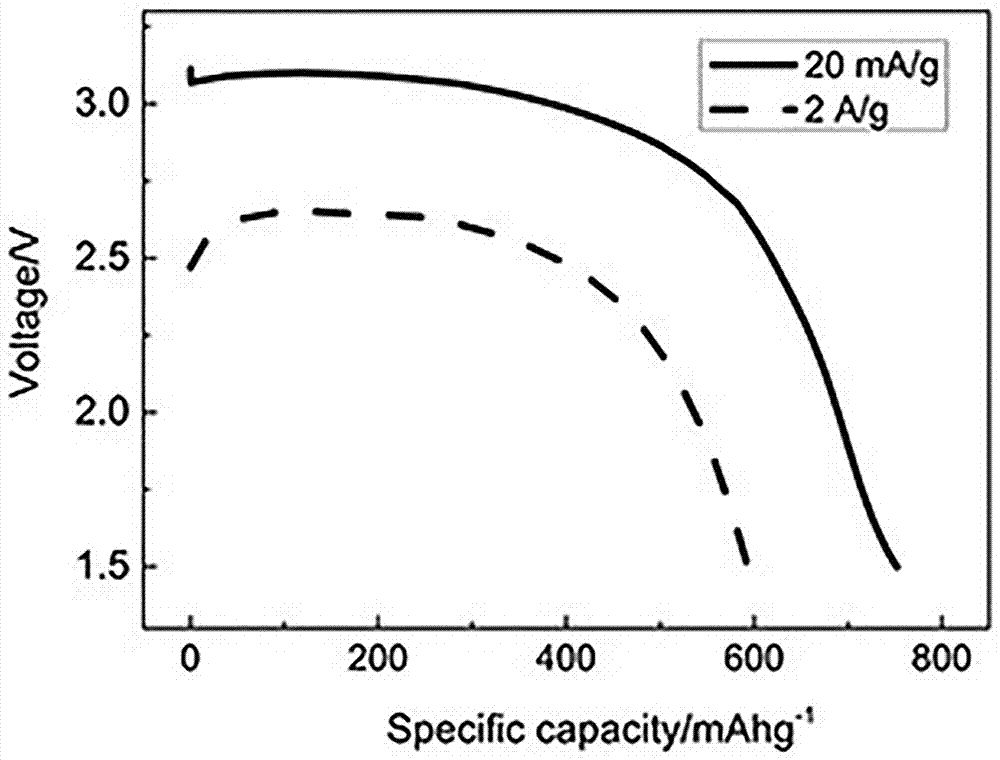
[0023] (2) The fluorination equipment was evacuated to make the vacuum degree reach -0.01 MPa, the fluorinated gas nitrogen trifluoride was introduced, the pressure was maintained at 200 kPa, and the reaction was carried out at 450 °C for 12 h to obtain 13.9 g of carbon fluoride. , in which the C content is 57.6 %, the F content is 42.4 %, and the discharge voltage plateau is 3.1 V (vs. Li + / Li).
Embodiment 2
[0025] (1) Put 10g of carbon powder into the fluorination equipment, the C content is 85%, the N content is 8%, the H content is 4%, and the O content is 3%;
[0026] (2) The fluorination equipment was evacuated to make the vacuum degree reach -0.01 MPa, the fluorinated gas nitrogen trifluoride was introduced, the pressure was kept at 300 kPa, and the reaction was carried out at 550 °C for 16 h to obtain 17.4 g of carbon fluoride. , in which the C content is 48.8%, the F content is 51.2%, and the discharge voltage plateau is 3.0 V (vs. Li + / Li).
Embodiment 3
[0028] (1) Put 10g of carbon powder into the fluorination equipment, the C content is 94%, the N content is 4%, and the H content is 2%;
[0029] (2) The fluorination equipment was evacuated to make the vacuum degree reach -0.01 MPa, the fluorinated gas nitrogen trifluoride was introduced, the pressure was maintained at 400 kPa, and the reaction was carried out at 600 °C for 16 h to obtain 21.3 g of carbon fluoride. , in which the C content is 44.1%, the F content is 55.9%, and the discharge voltage plateau is 3.0 V (vs. Li + / Li).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com