Alogliptin salt and its crystal form, their preparation method and pharmaceutical composition
A crystal form and crystallization technology, applied in the field of medicinal chemical crystallization, can solve the problems of poor crystal form stability, slow dissolution rate, and low water solubility, and achieve the effects of good crystal form stability, good solubility, and fast dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
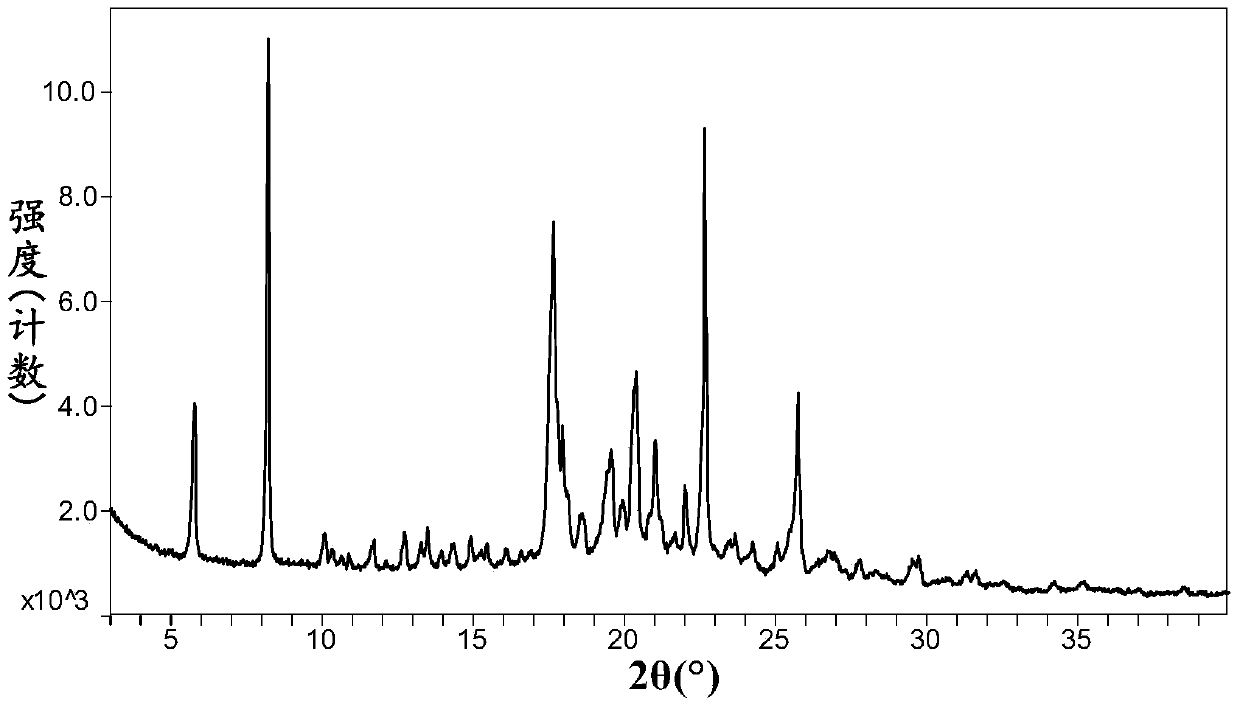
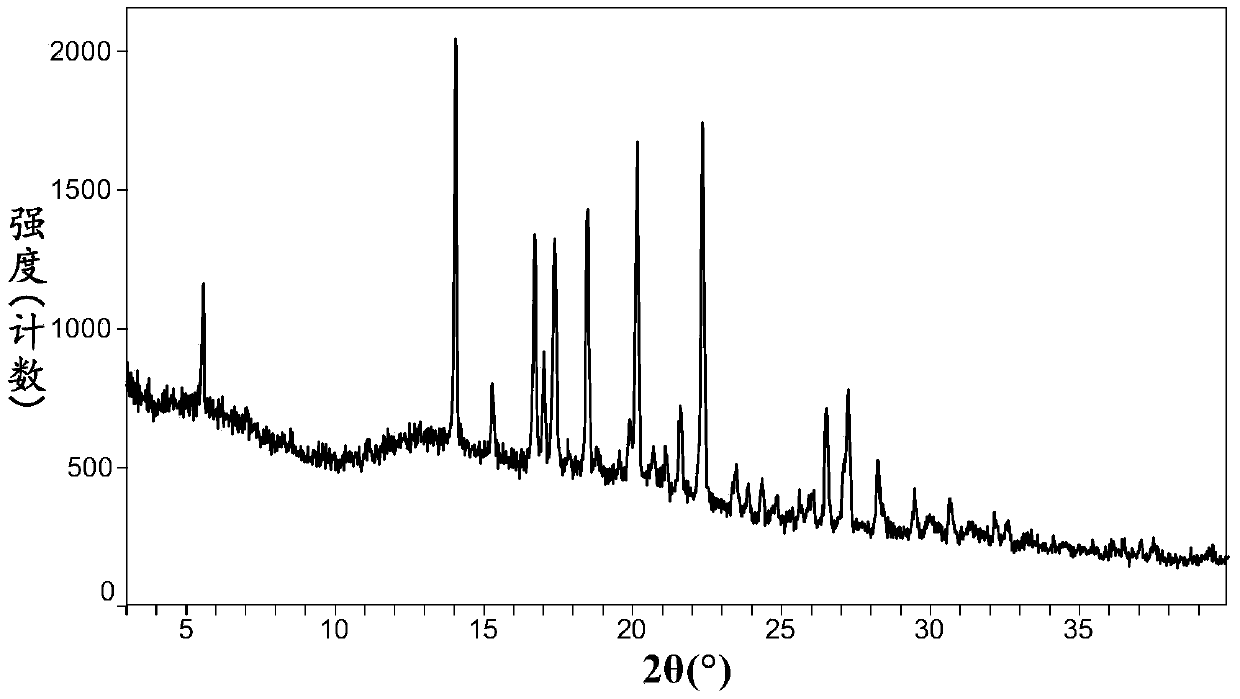
Image
Examples
preparation example 1
[0120] Preparation Example 1 Preparation of alogliptin
[0121] Prepared with reference to the preparation method of Patent Document CN103702562A Example 7, the specific operations are as follows:
[0122]
[0123] 1) 8.25g of ketone, 9.8g of pyrazole salt and 124mL of DMAc were charged into a 500mL three-necked flask (equipped with overhead stirring, N 2 inlet and thermocouple), and the resulting homogeneous solution was cooled to Ti = -10 °C. 6.94 g NaBH(OAc) was added portionwise as solid 3 . The reaction is aged at Ti=-10°C until the ketone consumption meets the requirement of ≥98%. NH via slow addition 4 OH (8.3ml) and H 2 Compound O (16.5ml) quenched the reaction slurry. The resulting slurry was heated to Ti = 50°C and then cooled to Ti = 22°C. Filter the slurry. Filter cake with 5:1 DMAc:H 2 O (65 mL) was used for washing, followed by water 65 mL displacement washing. The filter cake was dried until the amount of residual water was ≤ 10%. 10.6 g of the r...
Embodiment 1
[0127] Example 1 Preparation of alogliptin maleate
[0128] At room temperature, dissolve 500 mg of alogliptin in 20 mL of methanol, dissolve 146 mg of maleic acid in 5 mL of methanol, mix the methanol solution of maleic acid and the methanol solution of alogliptin, stir to form a slurry, filter, and filter cake at room temperature Drying under reduced pressure for 4 hours gave 452 mg of alogliptin maleate.
Embodiment 2
[0129] Example 2 Preparation of alogliptin maleate crystal form 1
[0130] At room temperature, 100 mg of augliptin maleate in Example 1 was added to 2 mL of isopropanol to form a suspension, stirred and crystallized for 16 hours, filtered, and the filter cake was dried under reduced pressure at room temperature for 16 hours to obtain 78.6 mg of augliptin Tine maleate crystal form 1, yield 78.6%.
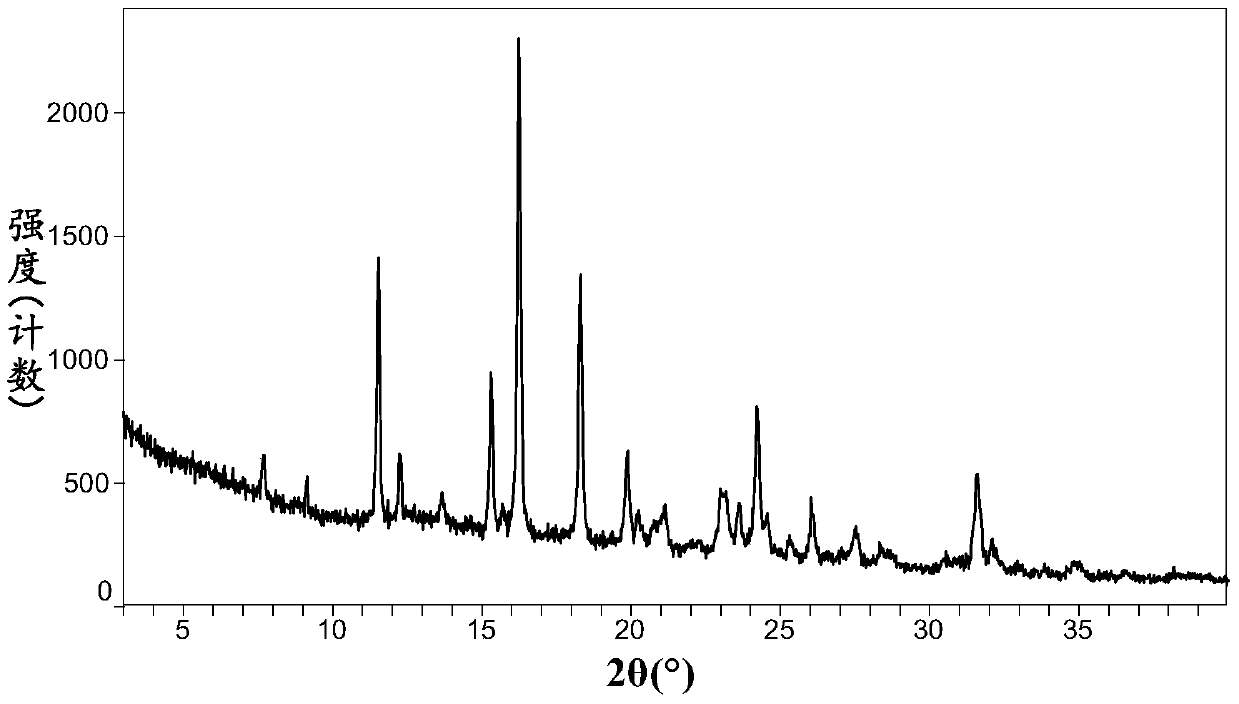
[0131] XRPD patterns such as figure 1 As shown, it shows a crystalline state, indicating that alogliptin maleate crystal form 1 has a good crystalline state.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com