Preparation method of targeted small molecular prodrug for pH response and collaborative treatment
A technology of small molecules and prodrugs, which is applied in the field of preparation of targeted amphiphilic small molecule prodrugs, can solve the problems of uncontrollable drug release, high content of anti-tumor drug carriers, and low drug loading capacity, so as to improve the dual-drug synergistic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
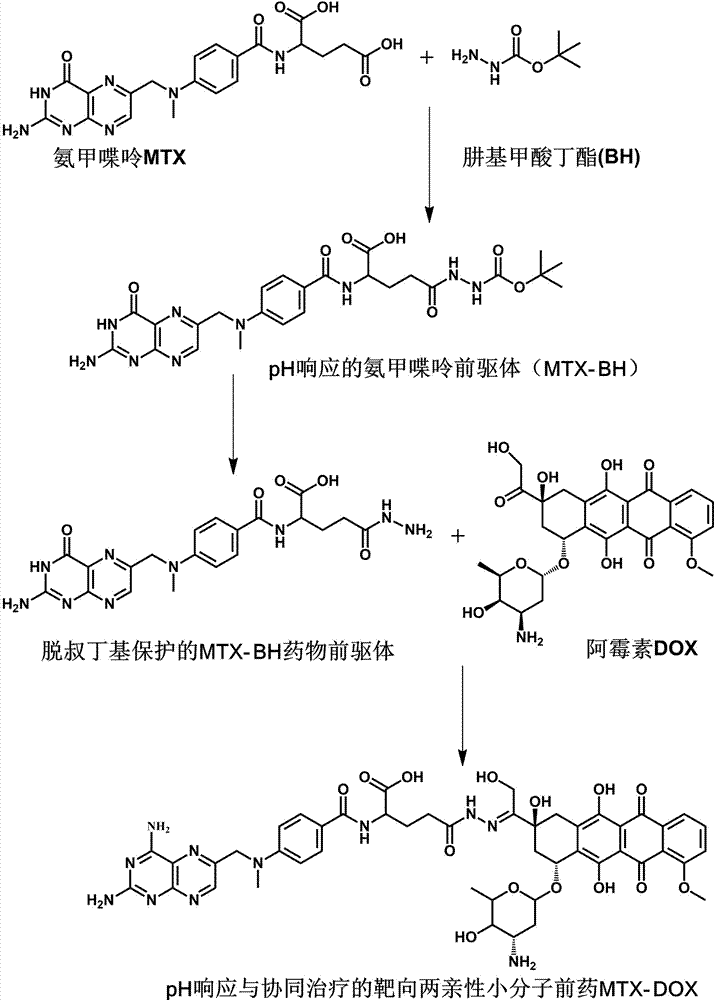
[0027] Example 1 Preparation of MTX-DOX Amphiphilic Small Molecule Prodrugs Responsive to pH Stimulation
[0028] (1) Under the condition of ice bath and argon (Ar) atmosphere, first dissolve 1.034 g MTX in 25 mL anhydrous N,N-dimethylformamide (DMF), then add 260.5 mg N-hydroxybutyrate Diimide (NHS) and 447.0 mg 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl); after stirring in an ice-water bath for 30 min, 325.3 mg Butyl carbazate (BH) was dissolved in 5 mL of anhydrous DMF solution, added to the above reaction solution, and stirred at room temperature in the dark for 24 h; precipitated in deionized water, filtered with suction and washed with acetone to obtain a stable product pH response The precursor of methotrexate (MTX-BH) was 308.0 mg, and the yield was 23.82%.
[0029] (2) First, the de-tert-butoxycarbonyl protection reaction of the amino group in MTX-BH, 85.4 mg of MTX-BH, a partial product of the reaction in step (1), was added to 1.5 mL of a m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com