Method of preparing fluorine-containing demoulding additive with industrial side product terpinene
A technology of by-product terpinene and mold release additives, which is applied in the direction of carboxylate preparation, carboxylic acid amide preparation, chemical instruments and methods, etc., can solve problems such as difficult to produce economic benefits and separation difficulties, and achieve sufficient raw material sources, Improved processing efficiency, simple and easy reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
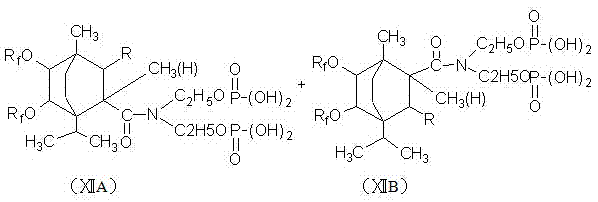
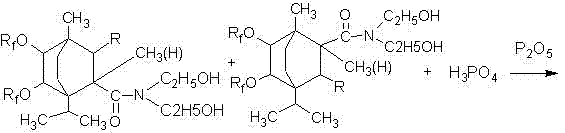
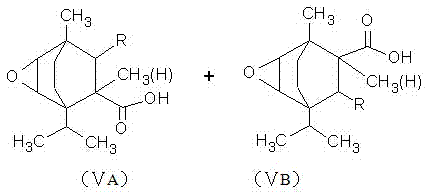
[0036] In a four-necked flask equipped with a stirring device, a reflux condensing device and a thermometer, add 30 grams of terpinene, 1.5 grams of toluenesulfonic acid and 0.5 grams of concentrated sulfuric acid, and after stirring for 0.5 to 1 hour, add 21 grams of 2-methanol Base-2-pentenoic acid, heat up to 170°C-180°C for reflux reaction for 5 hours to generate methylpentenoic acid terpinene addition compound, cool down to 60-70°C, add 25 grams of hydrogen peroxide, 5 grams Glacial acetic acid and 0.5 gram of concentrated sulfuric acid carried out epoxidation reaction for 4 hours to generate epoxidized 2-methyl-2-pentenoic acid terpinene addition compound, then hydrolyzed with 3 times the volume of hot water, and allowed to stand , take the oil layer to generate dihydroxyl products, add 150mL of toluene and 120g of hexafluoropropylene oligomer, start stirring, slowly add 1.5g of N,N-dimethylcyclohexylamine, and carry out prophylaxis at a temperature of 20°C to 35°C. Afte...
Embodiment 2
[0038] Add 30 grams of terpinene, 1.8 grams of p-toluenesulfonic acid and 1.2 grams of sulfamic acid into a four-neck flask equipped with a stirring device, a reflux condensing device and a thermometer, and after stirring for 0.5 to 1 hour, add 18 grams of crotonic acid , heated up to 150°C-160°C for reflux reaction for 4 hours to generate crotonyl terpinene addition compound, cooled to 65-75°C, added 21 grams of hydrogen peroxide, 5.5 grams of glacial acetic acid and 0.6 grams of concentrated sulfuric acid, and carried out Epoxidation reaction 3 hours, generate epoxidized butenoic acid terpinene addition compound, carry out hydrolysis with the hot water of 3 times volume then, leave standstill, get oily layer, generate double hydroxyl product, add 120mL toluene and 110 grams of six Fluoropropylene oligomer, start stirring, slowly add 2 grams of N,N-dimethylcyclohexylamine, carry out nucleophilic substitution reaction at a temperature of 20°C to 35°C for 6 to 8 hours, distill t...
Embodiment 3
[0040] In a four-neck flask equipped with a stirring device, a reflux condensing device and a thermometer, add 30 grams of terpinene, 1 gram of p-toluenesulfonic acid and 1.5 grams of sulfamic acid, stir for 0.5 to 1 hour, then add 24 grams of 2-hexane Acrylic acid, heat up to 160°C-170°C for reflux reaction for 6 hours to generate hexenoic acid terpinene addition compound, cool down to 70-80°C, add 24 grams of hydrogen peroxide, 6.5 grams of glacial acetic acid and 0.7 grams of concentrated sulfuric acid , carry out epoxidation reaction for 5 hours, generate epoxidized 2-hexenoic acid terpinene addition compound, then hydrolyze with 3 times the volume of hot water, let it stand, take the oil layer, generate dihydroxy product, add 100mL toluene and 105 grams of hexafluoropropylene oligomer, start stirring, slowly add 1 gram of N,N-dimethylcyclohexylamine, carry out nucleophilic substitution reaction at a temperature of 20°C to 35°C for 6 to 8 hours, and distill out toluene , to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com