Anti-HBV infection host HNF factor inhibitor and application thereof
A kind of use, anti-hepatitis B technology, applied in the specific inhibitor of host cell hepatic nuclear factor HNF, clinical treatment of hepatitis B virus infection disease, antiviral drug field, can solve the problems such as no literature reports, achieve the effect of enhancing the inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
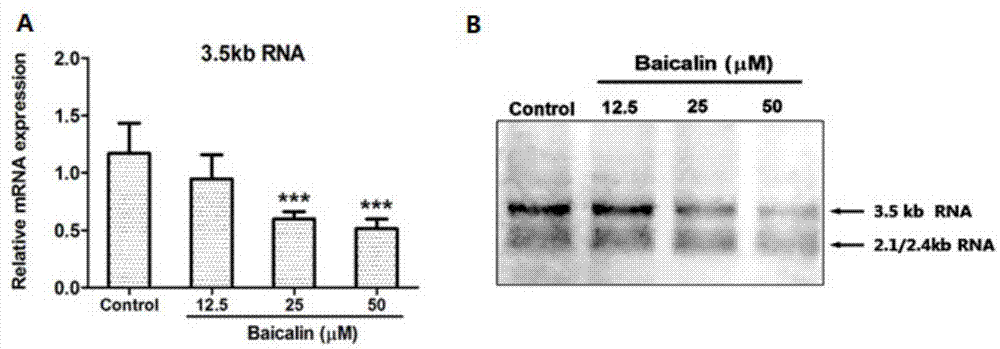
[0048] Baicalin (baicalin) inhibits liver nuclear factor HNF, down-regulates HBV-RNA level, reduces HBV virus surface antigen level and virus particle level test
[0049] After the HepG2 2.2.15 cells grow into a single layer, discard the culture medium, wash the cells once with EDTA solution, add 5 mL of digestive solution (EDTA solution: 0.5% trypsin = 5:1) to each bottle (25cm2 culture flask), and place at 37 ℃ incubator for 5-10 minutes, remove the digestive juice, add an appropriate amount of cell culture medium to each bottle, gently blow down and disperse the adherent cells with a pipette, divide the bottles for passage, and culture at 37°C, 5% CO2, 48 The medium was changed after 1 hour, and the cells were subcultured every 5 days on average. HepG2 2.2.15 cells were digested and prepared into 2×10 5 / mL cell suspension, inoculated in 48-well cell culture plates with 250 μL / well, after culturing for 48 hours to grow into a single layer of cells, add baicalin-containing ...
Embodiment 2
[0056] In vitro anti-HBV wild-type strain test of the combination of baicalin BA and entecavir ETV
[0057] HepG2 2.2.15 cells in 2×10 5 / mL density, 250μL / well was inoculated in a 48-well cell culture plate, and after culturing for 48 hours to grow into a monolayer of cells, a mixture of ETV and BA prepared with culture medium was added, respectively ETV3nM+BA 50μM, ETV0.75nM +BA 50 μM and ETV 0.19 nM +BA 50 μM. At the same time, ETV 3nM, 0.75nM and 0.19nM were used as ETV intervention control alone, and culture medium without drug was set as control. The culture was continued for another 9 days, during which the medium was changed every 3 days, and the cell supernatant was collected after 9 days of sample intervention, and the levels of HBsAg, HBeAg and HBV DNA were detected;
[0058] The detection of HBV antigen in the supernatant of HepG2 2.2.15 cells showed that the inhibitory effect of ETV on HBV antigen was weak when used alone, and the inhibitory effect was improved t...
Embodiment 3
[0060] Anti-HBV rtM204V+rtL180M mutant activity detection of entecavir baicalin combination in vitro
[0061] HepG2 cells were treated with 2×10 5 / mL cell density, 250 μL / hole was inoculated in a 48-well cell culture plate, cultivated for 24 hours, when about 80% confluence, transfected mutant plasmid rtM204V+rtL180M, then added the mixed solution of ETV and BA prepared with culture medium, They were ETV 48nM+BA 50μM, ETV 12nM+BA 50μM, ETV 3nM+BA 50μM and ETV 0.75nM+BA50μM. At the same time, the ETV concentration was 48nM, 12nM, 3nM and 0.75nM respectively. Drug culture solution control; after 6 days of sample intervention (during which the medium was changed once every other day), the cell supernatant was collected, and ELISA kits were used to detect HBsAg and HBeAg, and fluorescent quantitative PCR was used to detect HBV DNA levels;
[0062] The detection of HBV antigen in the supernatant of the HBV rtM204V+rtL180M drug-resistant mutant cell model showed that the inhibitor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com