Cimicifuga extract and two Cimicifone bases and preparation method and use
A technology of Cimicifume and Cimicifume B, which is applied in the field of medicine, can solve problems such as no reports on anti-tumor cytotoxic activity of Cimicifuga plant alkaloids, and achieve the effect of expanding the effect of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 10 kg of dried rhizomes of Cimicifuga yunnanensis were powdered and extracted twice with 80L of 95% ethanol solution at room temperature for 5 days each time. Combine the extracts and steam until there is no alcohol smell to obtain 1000 g of the total extract. The total extract was dispersed with 2 L of water, extracted with equal volumes of petroleum ether and ethyl acetate, respectively, to obtain the ethyl acetate extracted fraction CRE (530 g). The ethyl acetate extraction part was eluted with a gradient of ethanol on a macroporous resin (D101) chromatographic column (30%-95%), and 85%-95% of the eluted part was collected and dried to obtain 20 g of Cimicifuga japonicus extract.
Embodiment 2
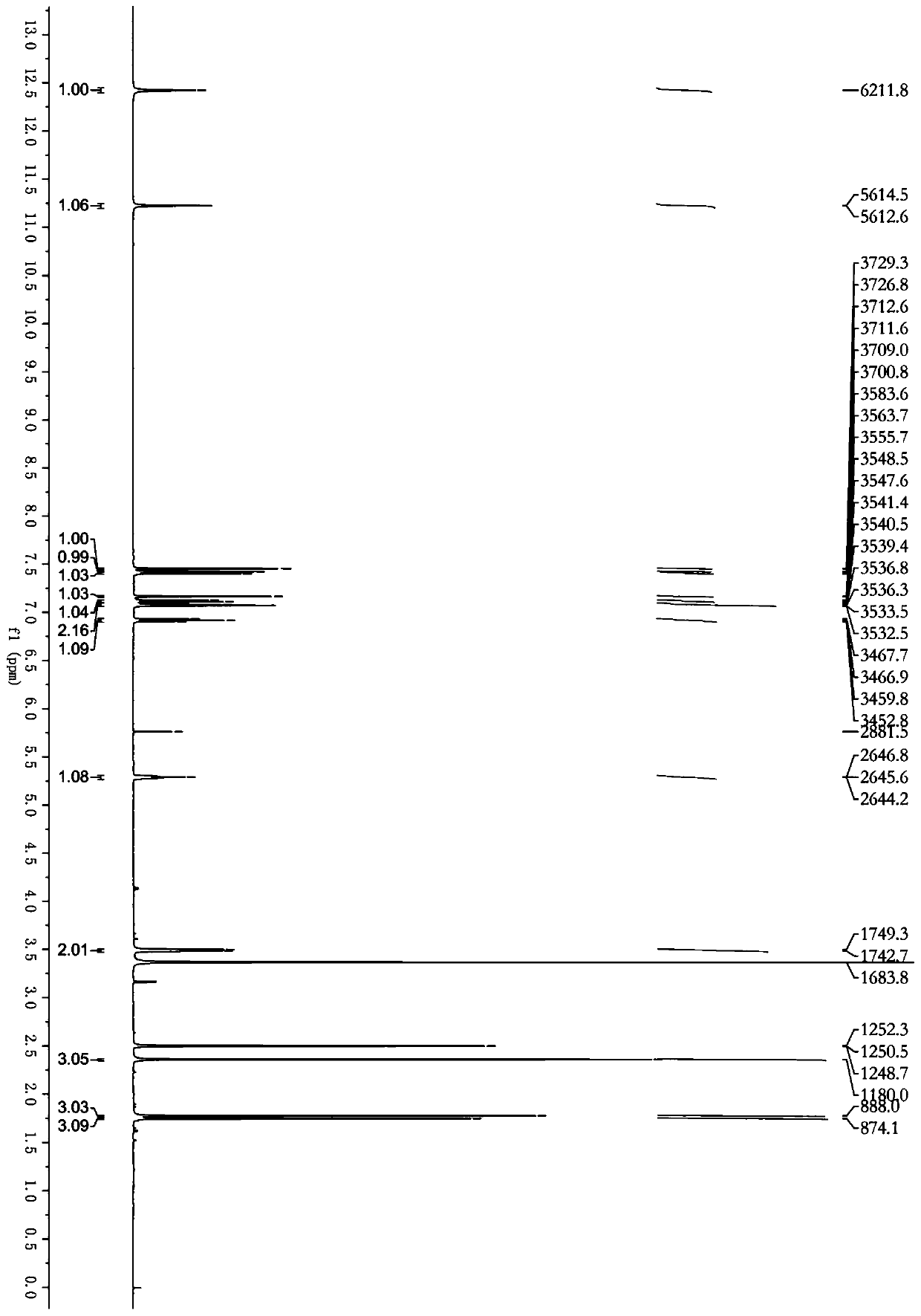
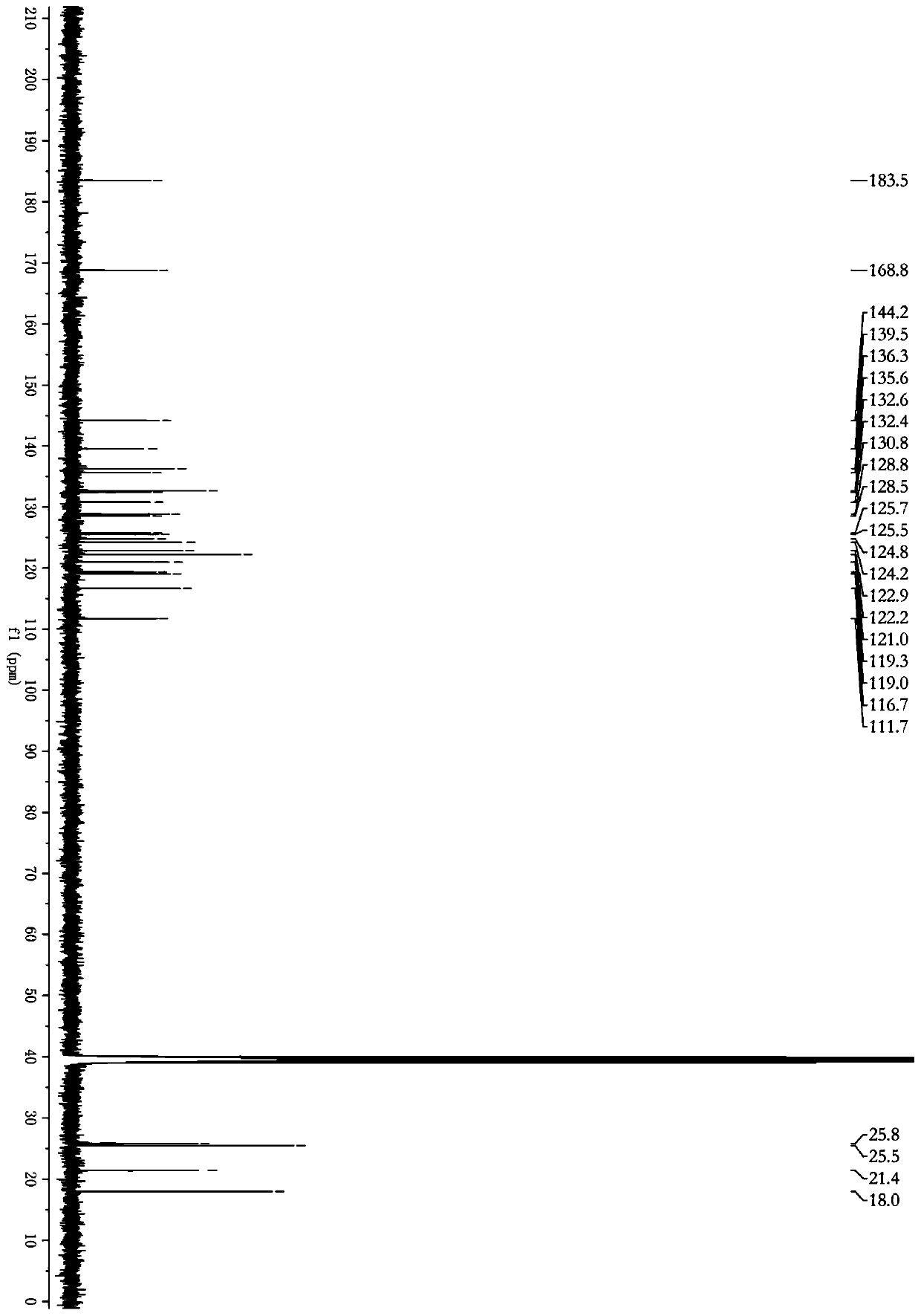
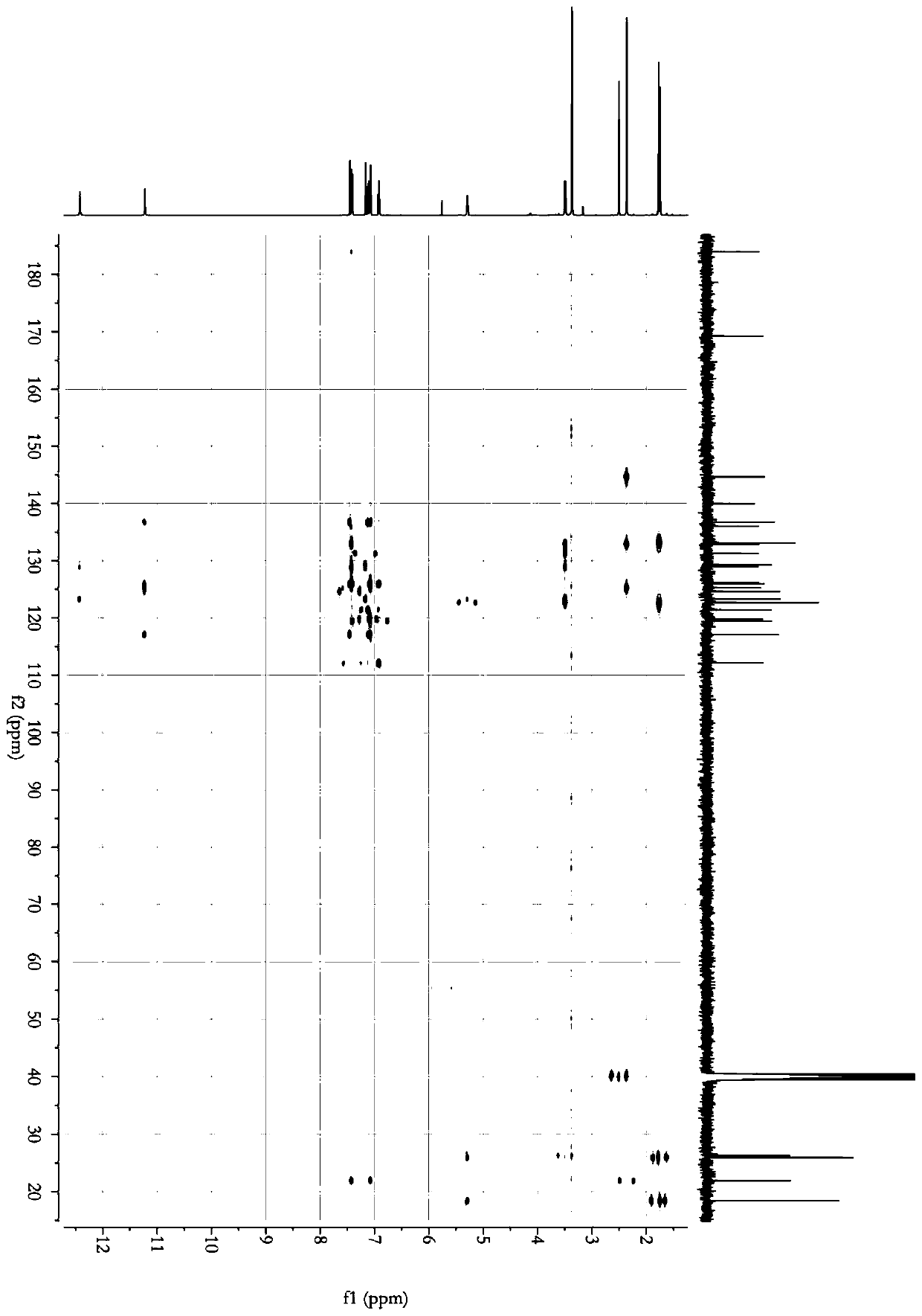
[0032]Dry 5 kg of Cimicifuga rhizome, powder it, and extract it twice with 50 L of ethyl acetate solution at room temperature for 3 days each time. Combine the extracts and steam until there is no alcohol smell to obtain 200 g of the total extract. The total extract was dispersed with 2L of water, and extracted with equal volumes of petroleum ether and ethyl acetate, respectively, to obtain the ethyl acetate extracted fraction CRE (100 g). The ethyl acetate extraction part was eluted with a macroporous resin (D101) chromatographic column (30%-95%) ethanol gradient, and the 80%-95% eluted part was collected to obtain the Cimicifuga japonicus extract. Further separated by Sephadex LH-20 column chromatography, using dichloromethane / methanol as the mobile phase, separated to obtain two fractions, which were crystallized in methanol respectively to obtain compound 1 (15 mg) and compound 2 (17 mg).
Embodiment 3
[0034] 5 kg of dried North American black cohosh rhizomes were crushed and then added with 40L of methanol for reflux extraction twice. The first time was 2 hours. After the extract was filtered, 40L of methanol was added to continue the reflux extraction for 1 hour. The two extracts were combined and then concentrated under reduced pressure to obtain an extract, which was suspended and dissolved in 3 L of water, and extracted successively with equal volumes of dichloromethane to obtain 420 g of extract. After gradient elution with macroporous resin (AB-8) chromatographic column (30% ~ 95%) ethanol, collect 75%-95% eluted fraction. Further separation by Sephadex LH-20 column chromatography, with dichloromethane / methanol as mobile phase, separated into two fractions, which were crystallized in dichloromethane to obtain compound 1 (45 mg) and compound 2 (52 mg).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com