Method for comprehensively recovering valuable metal from arsenic containing smoke and synthesizing arsenic fixing ore through fractional crystallization method
A valuable metal, fractional crystallization technology, applied in the field of metallurgy, can solve the problems of low comprehensive recovery rate of valuable elements, limited arsenic product market, untreated sodium arsenate, etc., achieve small BET specific surface area and improve the quality of recycled products , the effect of low arsenic content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
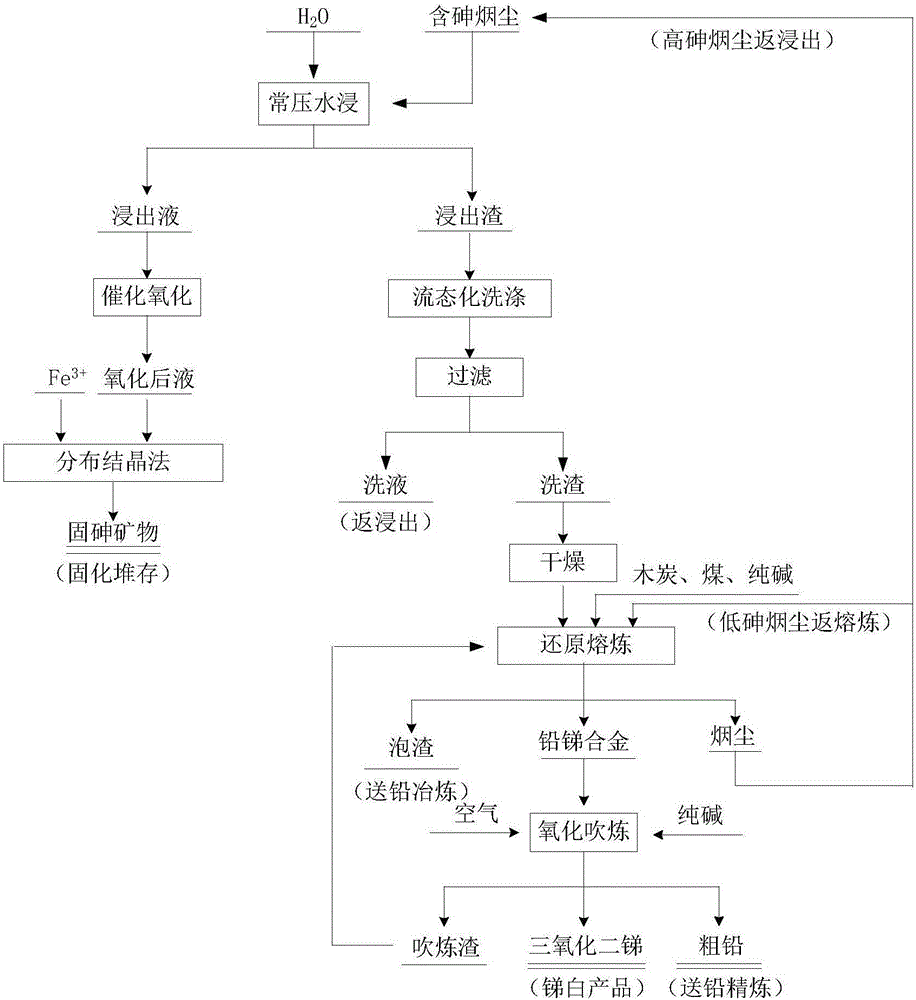
Image
Examples
Embodiment 1
[0041] Taking arsenic-containing soot from a lead-zinc smelter in China as an example, the main components of the raw materials are Pb 10.13%, As 30.11%, Sn 0.5%, Sb 30.02%, Zn 0.07%, Se 0.08%.
[0042] Proceed as follows:
[0043] (1) Weigh a certain amount of high-arsenic and antimony fumes in the reactor, and carry out the leaching experiment according to the liquid-solid volume-to-mass ratio of 10:1, the stirring speed of 700r / min, the leaching temperature of 80°C, and the leaching time of 2h. After the leaching is completed, remove the slurry and filter and separate it. The arsenic leaching rate is 51.25%. The concentration of each element in the leach solution is Pb 75.00ppm, Se 0.52ppm, Zn 48ppm, Sb 0.66g / L, As15.43g / L;
[0044] (2) The leaching liquid adopts the method of catalytic oxidation to convert As 3+ Oxidized to As 5+ , the control conditions are as follows: the oxygen flow rate is 5L / min, the As / Mn molar ratio is controlled at 10:1, and the temperature of th...
Embodiment 2
[0049] Taking arsenic-containing soot from a lead-zinc smelter in China as an example, the main components of the raw materials are Pb 8.64%, As 25.63%, Sn 0.58%, Sb 24.56%, Zn 0.09%, Se 0.10%.
[0050] Proceed as follows:
[0051] (1) Weigh a certain amount of high-arsenic-antimony fumes in the reaction kettle, and carry out the leaching experiment according to the liquid-solid volume-to-mass ratio of 5:1, the stirring speed of 300r / min, the leaching temperature of 60°C, and the leaching time of 2h. After leaching, remove the slurry and filter and separate, the arsenic leaching rate is 46.25%. The concentration of each element in the leach solution is Pb 69ppm, Se 0.73ppm, Zn 50ppm, Sb 0.67g / L, As11.85g / L;
[0052] (2) The leaching liquid adopts the method of catalytic oxidation to convert As 3+ Oxidized to As 5+ , the control conditions are as follows: the oxygen flow rate is 10L / min, the As / Mn molar ratio is controlled at 40:1, and the temperature of the catalytic oxidati...
Embodiment 3
[0057] Taking arsenic-containing soot from a lead-zinc smelter in China as an example, the main components of the raw materials are Pb 13.24%, As 29.31%, Sn 0.9%, Sb 27.68%, Zn 0.04%, Se 0.11%.
[0058] Proceed as follows:
[0059] (1) Weigh a certain amount of high-arsenic-antimony fumes into the reactor, and carry out the leaching experiment according to the liquid-solid volume-to-mass ratio of 15:1, stirring speed of 50r / min, leaching temperature of 40°C, and leaching time of 3h. After leaching, remove the slurry and filter and separate, the arsenic leaching rate is 42.35%. The concentration of each element in the leach solution is Pb 113ppm, Se 0.50ppm, Zn 92ppm, Sb 1.37g / L, As12.41g / L;
[0060] (2) The leaching liquid adopts the method of catalytic oxidation to convert As 3+ Oxidized to As 5+ , the control conditions are as follows: the oxygen flow rate is 1L / min, the As / Mn molar ratio is controlled at 20:1, and the temperature of the catalytic oxidation system is contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com