Preparation of pH-response doxorubicin-dopamine conjugate and prodrug nano particle thereof
A nanoparticle and doxorubicin technology, which is applied in the preparation of sugar derivatives, medical preparations of non-active ingredients, wave energy or particle radiation treatment materials, etc., can solve the problem of difficult application without doxorubicin-dopamine conjugates , Uncontrollable drug burst release and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
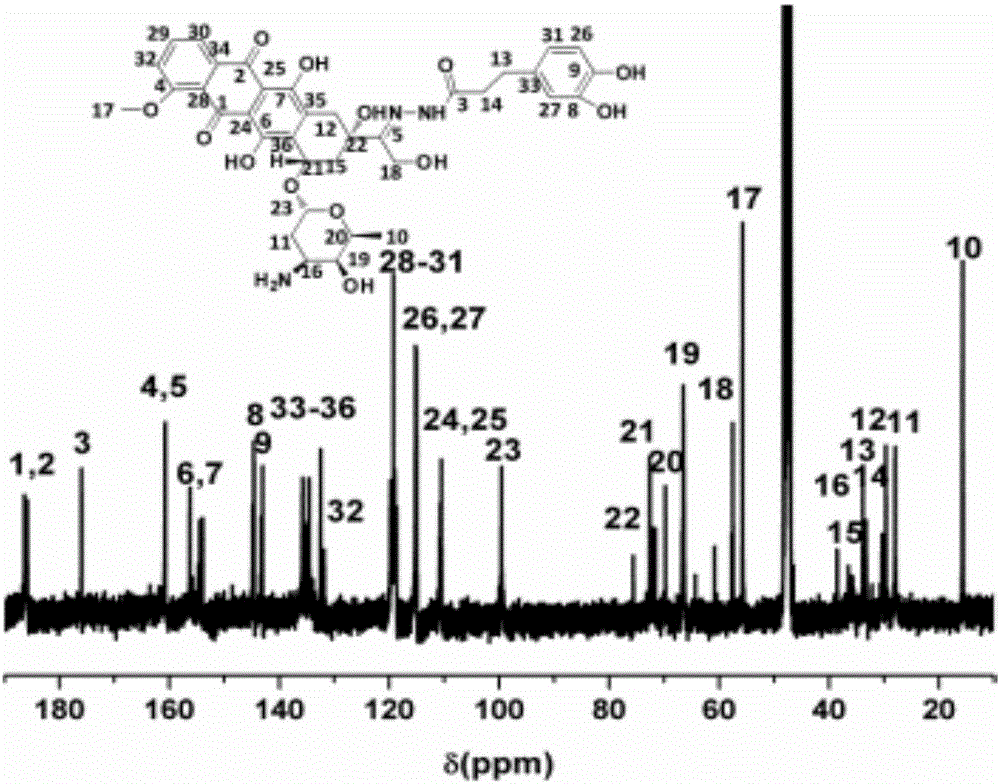
[0038] Embodiment 1 Preparation of doxorubicin-dopamine conjugate molecule
[0039] Step 1, 455.5 mg of 3,4-dihydroxyphenylpropionic acid, 405.36 mg of 1-hydroxybenzotriazole, and 619.2 mg of dicyclohexylcarbodiimide were dissolved in 20 mL of anhydrous dimethylformamide, After reacting at 25° C. for 4 hours, a dimethylformamide solution of 396.5 mg tert-butyl carbazate was added dropwise into the reaction flask. After reacting at 25°C for 20 hours, centrifuge to remove dicyclohexylurea, and then evaporate with an oil pump at 60°C. The column was loaded with petroleum ether, and the eluent was a mixed solvent of ethyl acetate:petroleum ether (3:1), Rf=0.35. Vacuum oven dry. The product is white powdery solid tert-butyl-2-(3-(3,4-dihydroxyphenyl)propionyl)hydrazinecarboxylate. The yield is 47.3-61.0%.
[0040]Step 2: Dissolve tert-butyl-2-(3-(3,4-dihydroxyphenyl) propionyl) hydrazine carboxylate in 5 mL of dichloromethane, slowly add 2 mL of trifluoroacetic acid, and stir a...
Embodiment 2p
[0045] Example 2 Preparation of pH-responsive doxorubicin-polydopamine prodrug nanoparticles
[0046] Add 410mg of tris, 20mL of distilled water to a 50mL round bottom flask, stir at 30°C for 30 minutes, dissolve 12.5mg of dopamine hydrochloride and 2.74mg of the doxorubicin-dopamine conjugate molecules obtained in Example 1 in 1mL of distilled water was quickly injected into the above solution, and the reaction time was 24 hours, and the nanoparticle solution was black. Distilled water dialysis (dialysis bag molecular weight cut-off 3500) for two days, 1000mL distilled water × 8. Freeze dry for 48 hours. The yield is 38%~45%.
[0047] The dynamic light scattering pattern of the doxorubicin-polydopamine prodrug nanoparticles prepared in this embodiment is as follows: Figure 4 As shown, its number average particle diameter is 90±8nm, and PDI is 0.65±0.02.
Embodiment 3p
[0048] Example 3 Preparation of pH-responsive doxorubicin-polydopamine prodrug nanoparticles
[0049] The difference between this example and Example 2 is that the molecular weights of dopamine hydrochloride and doxorubicin-dopamine conjugate are 12.5 mg and 5 mg, respectively. The yield of the product obtained in this example is 34%-39%.
[0050] The dynamic light scattering pattern of the doxorubicin-polydopamine prodrug nanoparticles prepared in this embodiment is as follows: Figure 5 As shown, the number average particle diameter is 88±8nm, and the PDI is 0.46±0.08.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com