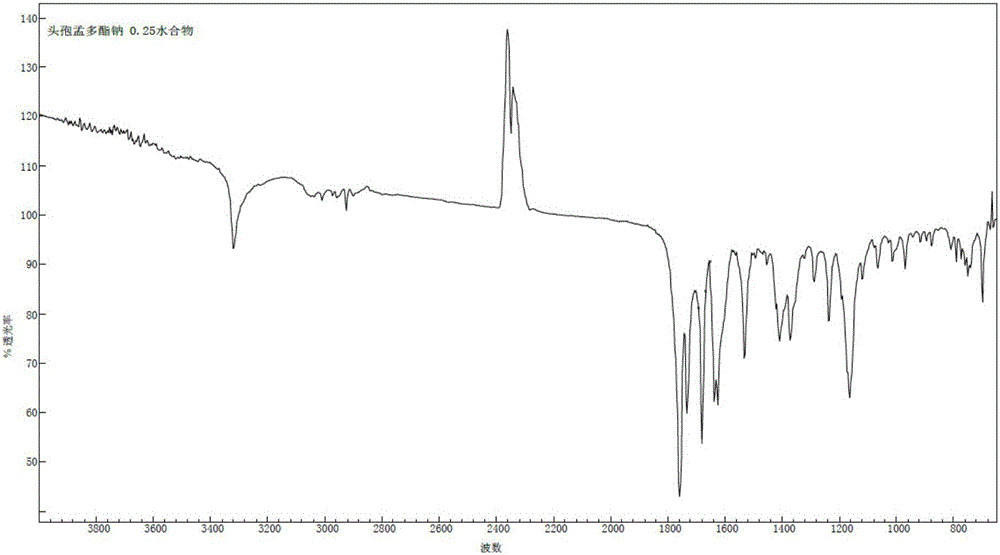
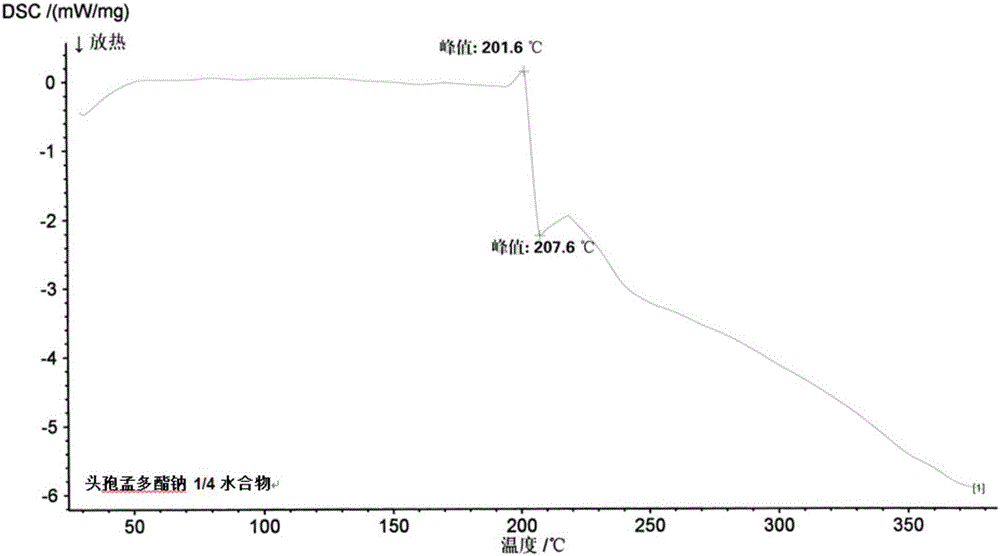
1/4 water and cefamandole nafate compound
A technology of cefamandide sodium and montdol sodium, which is applied in the directions of organic chemistry, antibacterial drugs, organic active ingredients, etc., can solve the problems of unqualified turbidity, low thermal decomposition temperature, poor fluidity, etc., and achieve product quality Stable, wide application prospects, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 1 / 4 cefamandole sodium compound
[0026] (1) 1L acetonitrile, 200g 3-acetoxymethyl-5-sulfur-7-amino-8-oxo-1-azabicyclooct-2-ene-2carboxylic acid, 1kg methylmercaptotetrazolium, Mix 900ml of boron trifluoride / acetonitrile complex, heat up to 30°C, stir rapidly for 2 hours, cool down to 0°C after the reaction, mix the reaction liquid with 1.5L of purified water pre-cooled to 0°C, and stir slowly until After crystals are precipitated, stir for another 30 minutes, add 10% ammonia water to adjust the pH value to 3.0, control the temperature at 0-5° C., grow the crystals for 2 hours, filter, wash twice with 1 L of acetone, and dry in vacuum to obtain a white crystalline powder (6R ,7R)-7amino-3[(Z)-2-(4-methylthiazol)-5-yl]vinyl-8oxo-5-thia-1-azabicyclo[4.2.0]octane -2-ene-2-carboxylic acid (223.5 g);
[0027] (2) (6R,7R)-7amino-3[(Z)-2-(4-methylthiazol)-5-yl]vinyl-8oxo-5-thia-1-azabicyclo [4.2.0] Mix oct-2-ene-2-carboxylic acid with 2L of e...
Embodiment 2
[0029] The preparation of embodiment 2 1 / 4 cefamandole sodium compound
[0030] (1) Mix 1.5L acetonitrile, 300g 3-acetoxymethyl-5-sulfur-7-amino-8-oxo-1-azabicyclooct-2-ene-2carboxylic acid, 1.5kg methylmercaptotetraazole Mix oxazole and 1.4L boron trifluoride / acetonitrile complex, raise the temperature to 40°C, stir the reaction rapidly for 1h, cool down to 5°C after the reaction, and mix the reaction solution with 2.25L purified water pre-cooled to 5°C, Stir slowly until crystals are precipitated, then stir for 30 minutes, add 10% ammonia water to adjust the pH to 3.4, control the temperature at 0-5°C, grow crystals for 3 hours, filter, wash twice with 1.5L of acetone, and dry in vacuum to obtain white crystals Sexual powder (6R,7R)-7amino-3[(Z)-2-(4-methylthiazol)-5-yl]vinyl-8oxo-5-thia-1-azabicyclo[4.2 .0] Oct-2-ene-2-carboxylic acid (319.6 g);
[0031] (2) (6R,7R)-7amino-3[(Z)-2-(4-methylthiazol)-5-yl]vinyl-8oxo-5-thia-1-azabicyclo [4.2.0] Mix oct-2-ene-2-carboxylic ac...
Embodiment 3
[0033] The preparation of embodiment 3 1 / 4 cefamandole sodium compound
[0034] (1) 1.25L acetonitrile, 250g 3-acetoxymethyl-5-sulfur-7-amino-8-oxo-1-azabicyclooct-2-ene-2 carboxylic acid, 1.25kg methylmercaptotetraazole Mix oxazole and 1.17L boron trifluoride / acetonitrile complex, heat up to 35°C, stir rapidly for 2 hours, cool down to 3°C after the reaction, mix the reaction solution with 1.88L purified water pre-cooled to 2°C, Stir slowly until crystals are precipitated, then stir for 30 minutes, add 10% ammonia water to adjust the pH to 3.2, control the temperature at 0-5°C, grow crystals for 2 hours, filter, wash twice with 1.3L of acetone, and dry in vacuum to obtain white crystals Sexual powder (6R,7R)-7amino-3[(Z)-2-(4-methylthiazol)-5-yl]vinyl-8oxo-5-thia-1-azabicyclo[4.2 .0] Oct-2-ene-2-carboxylic acid (261.1 g);
[0035] (2) (6R,7R)-7amino-3[(Z)-2-(4-methylthiazol)-5-yl]vinyl-8oxo-5-thia-1-azabicyclo [4.2.0] Mix oct-2-ene-2-carboxylic acid with 2.5L ethyl aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com