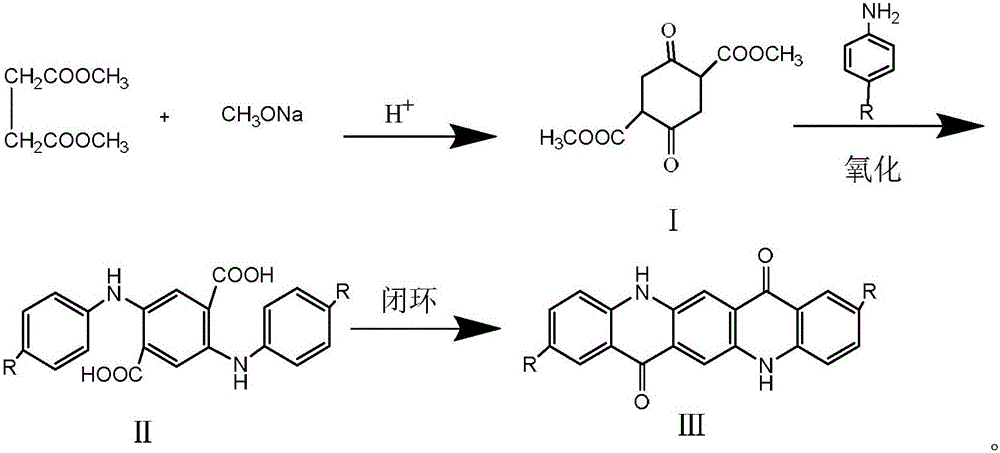
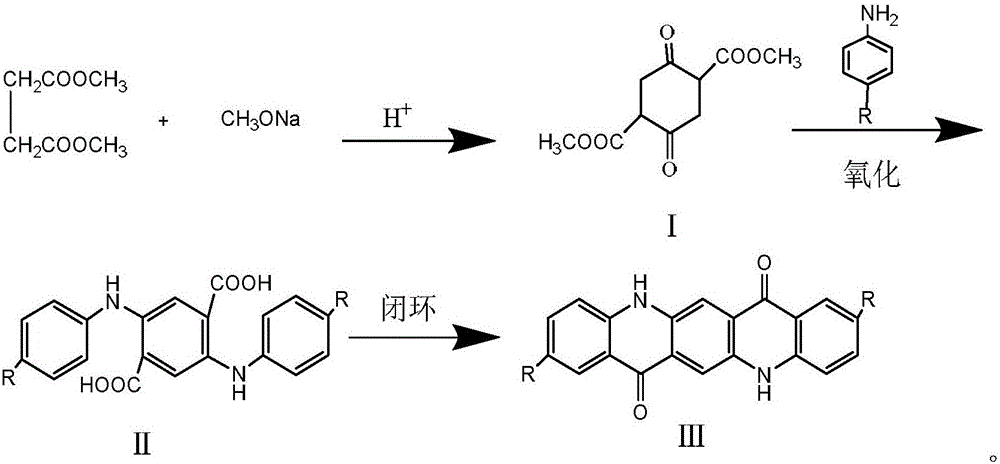
Preparation method for quinacridone and derivatives of quinacridone
A technology for quinacridone and derivatives, which is applied in the field of preparation of quinacridone and derivatives thereof, can solve the problems of large size, large environmental pollution and high energy consumption, and achieves mild oxidation temperature conditions, good effect and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1, the preparation of dimethyl succinyl succinate (DMSS):
[0032] In a 1L three-necked flask equipped with an electric heating magnetic stirrer, a thermocouple, a constant pressure dropping funnel and a condenser tube (decompression distillation device) were installed. In a three-necked flask, 600 g of dimethyl succinate DMS (4 mol) was added, and the temperature was raised to 100° C. under the protection of nitrogen (moisture and oxygen were removed). Afterwards, 180 g of 30% sodium methoxide solution (1 mol) was slowly added dropwise with a constant pressure dropping funnel, and the dropping time was about 4 hours. After the dropping was completed, the reaction was continued for about 2 hours. After the reaction, the product was obtained, pickled, washed with water, and dried to obtain 86 g of a light yellow substance, dimethyl succinylate succinate DMSS, with a yield of 75.4% and a melting point of 154.6. 1 HNMR (400MHz, CDCl 3 ), δ / ppm: 1.67 (s, 3H, OCH 3 ), 3.3...
Embodiment 2
[0040] 1, the preparation of dimethyl succinyl succinate (DMSS):
[0041] In a 1L three-necked flask equipped with an electric heating magnetic stirrer, a thermocouple, a constant pressure dropping funnel and a condenser tube (decompression distillation device) were installed. In a three-necked flask, 600 g of dimethyl succinate DMS (4 mol) was added, and the temperature was raised to 100° C. under the protection of nitrogen (moisture and oxygen were removed). Afterwards, 180 g of 30% sodium methoxide solution (1 mol) was slowly added dropwise with a constant pressure dropping funnel, and the dropping time was about 4 hours. After the dropping was completed, the reaction was continued for about 2 hours. After the reaction, the product was obtained, pickled, washed with water, and dried to obtain 86 g of a light yellow substance, dimethyl succinylate succinate DMSS, with a yield of 75.4% and a melting point of 154.6. 1 HNMR (400MHz, CDCl 3 ), δ / ppm: 1.67 (s, 3H, OCH 3), 3.38...
Embodiment 3
[0049] 1, the preparation of dimethyl succinyl succinate (DMSS):
[0050] In a 1L three-necked flask equipped with an electric heating magnetic stirrer, a thermocouple, a constant pressure dropping funnel and a condenser tube (decompression distillation device) were installed. In a three-necked flask, 600 g of dimethyl succinate DMS (4 mol) was added, and the temperature was raised to 100° C. under the protection of nitrogen (moisture and oxygen were removed). Afterwards, 180 g of 30% sodium methoxide solution (1 mol) was slowly added dropwise with a constant pressure dropping funnel, and the dropping time was about 4 hours. After the dropping was completed, the reaction was continued for about 2 hours. After the reaction, the product was obtained, pickled, washed with water, and dried to obtain 86 g of a light yellow substance, dimethyl succinylate succinate DMSS, with a yield of 75.4% and a melting point of 154.6. 1 HNMR (400MHz, CDCl 3 ), δ / ppm: 1.67 (s, 3H, OCH 3 ), 3.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com