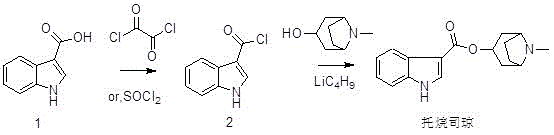
Preparation method of tropisetron hydrochloride
A technology for tropisetron hydrochloride and preparation steps, which is applied in the field of preparation of tropisetron hydrochloride, can solve problems such as expensive n-butyllithium, hidden dangers in production safety, and difficulty in evaporation to dryness, and saves workload and cost. , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
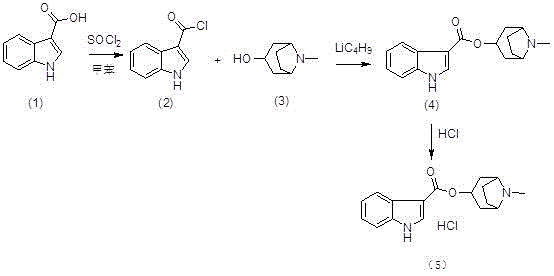
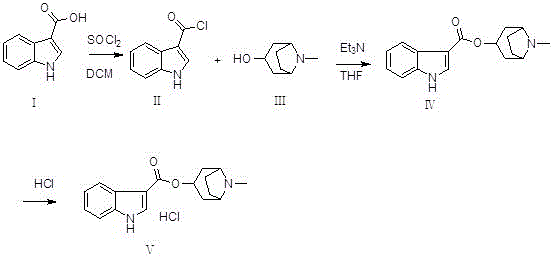
[0032] (1) Preparation of (II) ( Figure 4 ):
[0033] Add reactant (I) (50g, 0.31mol) and dichloromethane (300mL) into the reaction flask, cool down to about 0°C, then add thionyl chloride (27mL, 0.37mol) dropwise to the above reaction flask During the middle, the drop was completed, raised to room temperature and stirred for 2 hours, and TLC monitored the progress. After the reaction was complete, the reaction system was concentrated to dryness at room temperature under reduced pressure to obtain a yellow solid (II), regardless of weight and yield, which was dissolved by adding tetrahydrofuran (400mL). directly used in the next step reaction;
[0034] (2) Preparation of (IV) ( Figure 5 ):
[0035] Add (Ⅲ) (43.8g, 0.31mol), tetrahydrofuran (400mL), and triethylamine (64.5mL, 0.47mol) into the reaction flask, and add the tetrahydrofuran solution of 3-indolecarbonyl chloride prepared above to the reaction flask dropwise. In the bottle, after dropping, raise the temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com