Kit for detecting drug resistance of mycobacterium tuberculosis based on HRM technology and primers thereof
A Mycobacterium tuberculosis, technical detection technology, applied in the field of kits for detecting drug resistance of Mycobacterium tuberculosis, can solve the problems of low sensitivity and long detection period of drug resistance of Mycobacterium tuberculosis, and achieve high sensitivity , shorten the detection cost, and the effect of simple detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The design and synthesis of embodiment 1 primer
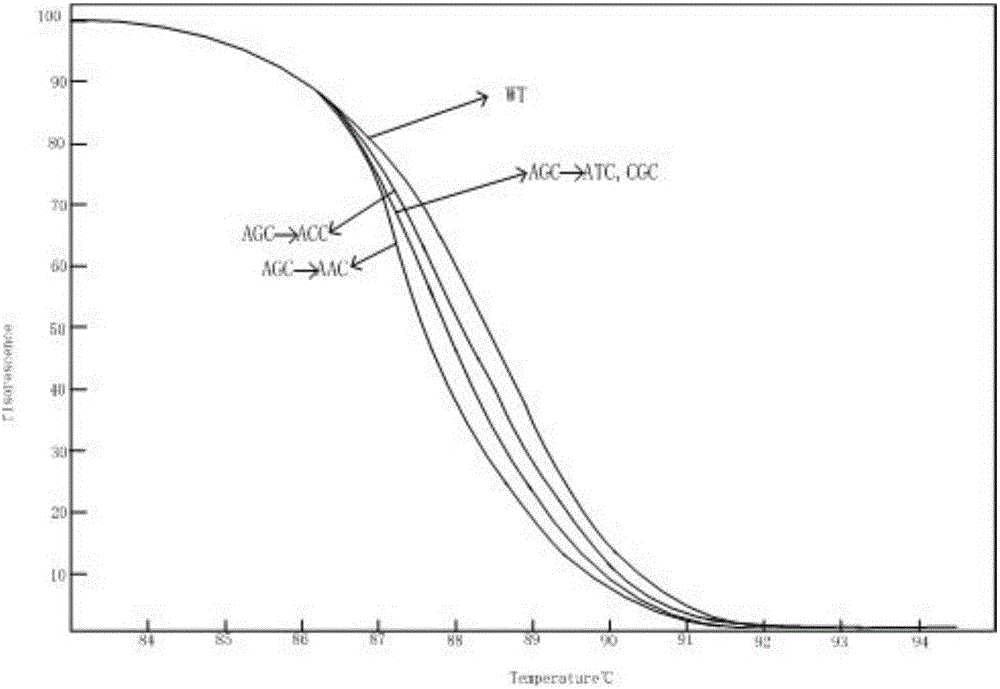
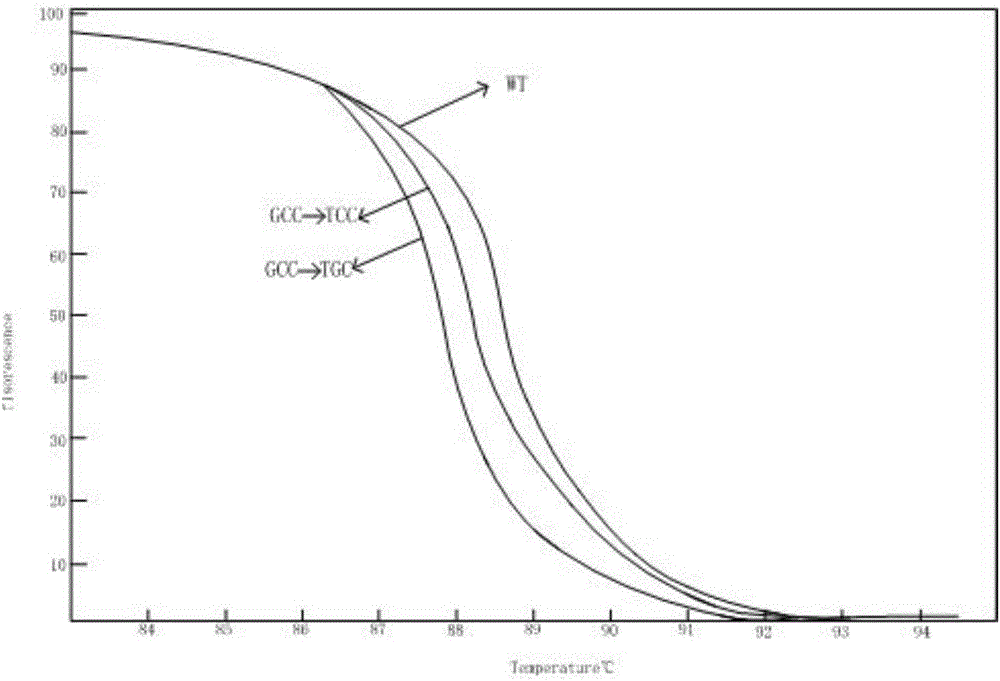
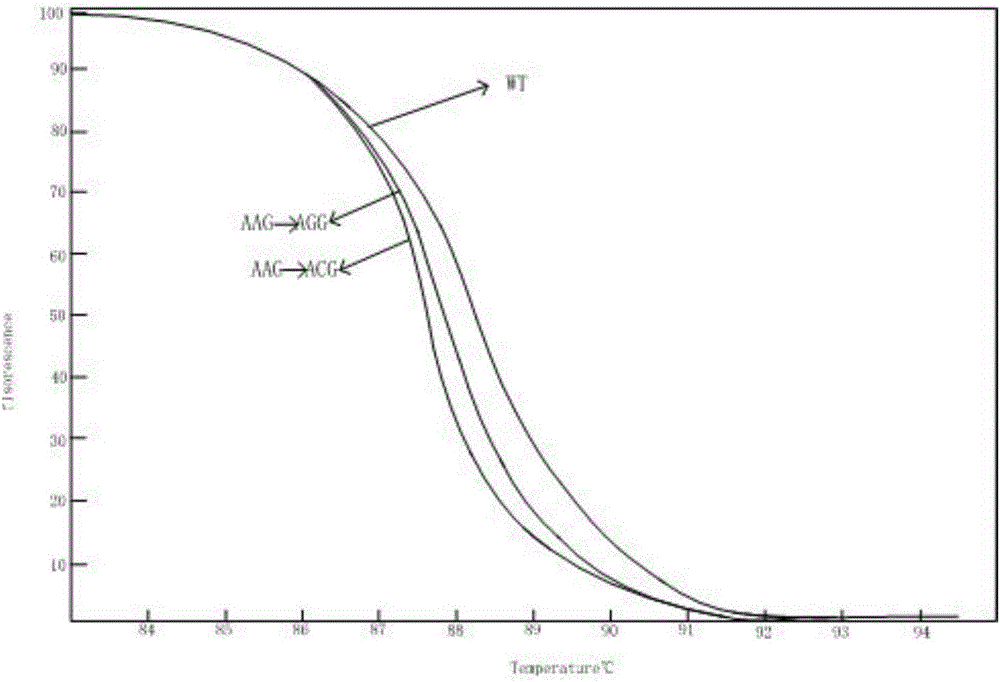
[0061] Combined with previous literature reports, the genes and corresponding mutation sites used to identify the multi-drug resistance of Mycobacterium tuberculosis were determined. The drug-resistant genes of Mycobacterium tuberculosis detected were: isoniazid resistance (katG gene and inhA gene), resistance Ethambutol (embB gene) and streptomycin resistance (rpsL gene). In NCBI, the standard sequences of katG, inhA, embB and rpsL can be obtained (the sequences are from the standard strain H37Rv, NC_000962.2), primers are designed upstream and downstream of the drug resistance mutation sites of the four genes, and the mutations of the four genes The location information, mutation type, primer sequence and amplified region are shown in Table 1:
[0062] Table 1 Mutation location information, mutation type, primer sequence and amplified region of the drug resistance gene of Mycobacterium tuberculosis
[0063]
Embodiment 2
[0064] The preparation of embodiment 2 kit
[0065] This embodiment provides a kit for detecting drug resistance of Mycobacterium tuberculosis based on HRM technology, said kit including the primers for detecting drug resistance gene mutation of Mycobacterium tuberculosis based on HRM technology in the above-mentioned Example 1.
[0066] The reaction solution (10 μl system, excluding DNA template) in the non-labeled fluorescent PCR amplification process included in the kit consists of the following components:
[0067] Green-2-Go qPCR Mastermix (including EvaGreen), 5μl;
[0068] Primer F, 10 μM, 0.1 μl;
[0069] Primer R, 10 μM, 0.1 μl;
[0070] wxya 2 O, 4.3 μl;
[0071] Also include negative standard and positive standard in the test kit, the preparation method of described standard comprises the steps:
[0072] 1. According to the gene variation of each tuberculosis drug-resistant gene wild type and drug-resistant type, in combination with the primers used in the pres...
Embodiment 3
[0092] The use of embodiment 3 kits
[0093] This embodiment provides an application for mutation detection at the predetermined site of the drug-resistant gene of Mycobacterium tuberculosis using the kit of Embodiment 2, including the following steps
[0094] (1) Collect the sputum sample for liquefaction and culture, extract the genomic DNA in the sputum sample, and prepare the DNA extract of the sputum sample:
[0095] 1) Transfer 1-2mL of sputum to a processing tube in a biological safety cabinet, add 1-4 times the volume of 4% sodium hydroxide solution, vortex for 30 seconds until fully liquefied, and let stand for 15 minutes;
[0096] 2) Add PBS to neutral, transfer to a 5ml centrifuge tube, centrifuge at 6480g for 15min, discard the supernatant;
[0097] 3) Add 0.5ml PBS to step 2) and mix evenly, draw 0.1ml with a syringe, inject it into a tuberculosis culture bottle and cultivate it for 42 days;
[0098] 4) Positive samples were transferred to Roche medium and used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com