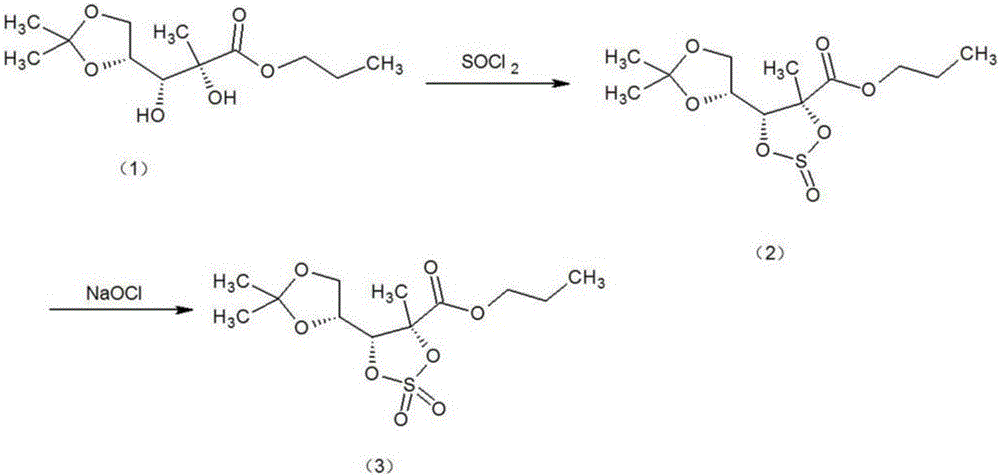
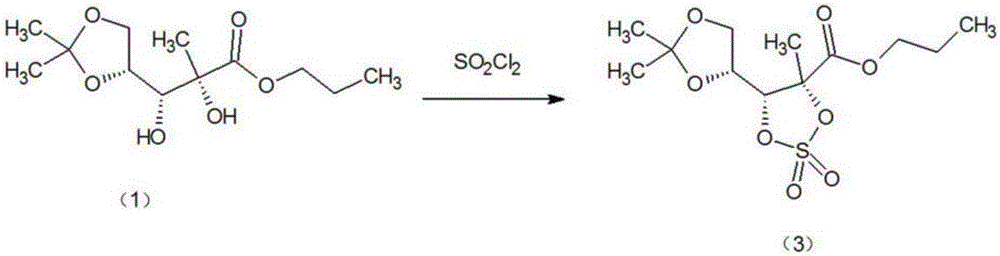
Method for preparing sofosbuvir intermediate through one-step process
An intermediate and step technology, applied in the field of one-step preparation of sofosbuvir intermediates, can solve the problems of long process route, poor product quality, difficult to handle and the like, and achieve the advantages of short process flow, good quality and reduced reaction steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] like figure 2 The synthetic route of the sofosbuvir intermediate shown, will add 300g dichloromethane, α-picoline 70g, raw material 2-C-methyl-4,5-O-(1- Methylvinyl)-D-ethyl arabinonic acid ester (1) 150g (0.57mol), cooled to below 15°C, slowly add 81g (0.60mol) of sulfuryl chloride dropwise under stirring condition and maintain 10-20°C, sulfuryl chloride After the dropwise addition, slowly raise the temperature to 20-25°C, and keep warm at this temperature for 8 hours, add 300ml of water to wash and separate the organic phase, dry the organic phase with anhydrous magnesium sulfate, and then concentrate to dryness to obtain the target product (3) , 171.2g (0.53mol), yield 92.98%, GC purity 99.16%. (GC Analysis Conditions Detector: Hydrogen Flame Ionization (FID) Detector; Chromatographic Column Model: DM-5, 30m0.25mmID0.25µm; Carrier Gas / Flow: Nitrogen / 0.1Mpa; Hydrogen: 0.1Mpa; Column Temperature: 150 ℃; Injector temperature: 180℃; Detector temperature: 190℃; Injecti...
Embodiment 2
[0019] like figure 2 The synthetic route of the sofosbuvir intermediate shown, will add 100g dichloromethane, α-picoline 25g, raw material 2-C-methyl-4,5-O-(1- Methylvinyl)-D-ethyl arabinonic acid ester (1) 50g (0.19mol), cooled to below 15°C, slowly add 31.0g (0.23mol) of sulfuryl chloride dropwise under stirring condition and maintain 10-20°C, sulfur After the addition of acid chloride, slowly raise the temperature to 25-30°C, and keep it at this temperature for 5 hours, add 100ml of water to wash and separate the organic phase, and then dry and concentrate the organic phase with anhydrous magnesium sulfate to obtain this product, 50.4g (0.18mol), yield 94.74%, GC purity 99.04%. (GC Analysis Conditions Detector: Hydrogen Flame Ionization (FID) Detector; Chromatographic Column Model: DM-5, 30m0.25mmID0.25µm; Carrier Gas / Flow: Nitrogen / 0.1Mpa; Hydrogen: 0.1Mpa; Column Temperature: 150 ℃; Injector temperature: 180℃; Detector temperature: 190℃; Injection volume: 0.5µl)
Embodiment 3
[0021] like figure 2 The synthetic route of the sofosbuvir intermediate shown, will add 250g dichloromethane, α-picoline 60g, raw material 2-C-methyl-4,5-O-(1- Methylvinyl)-D-ethyl arabinonic acid ester (1) 100g (0.38mol), cooled to below 15°C, slowly add 59.4g (0.44mol) of sulfuryl chloride dropwise under stirring condition and maintain 10-20°C, sulfur After the addition of the acid chloride, slowly raise the temperature to 15-20°C, and keep warm at this temperature for 10 hours, add 200ml of water to wash and separate the organic phase, and then dry and concentrate the organic phase with anhydrous magnesium sulfate to obtain this product, 113.5g (0.35mol), yield 92.10%, GC purity 99.31%. (GC Analysis Conditions Detector: Hydrogen Flame Ionization (FID) Detector; Chromatographic Column Model: DM-5, 30m0.25mmID0.25µm; Carrier Gas / Flow: Nitrogen / 0.1Mpa; Hydrogen: 0.1Mpa; Column Temperature: 150 ℃; Injector temperature: 180℃; Detector temperature: 190℃; Injection volume: 0.5µ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com