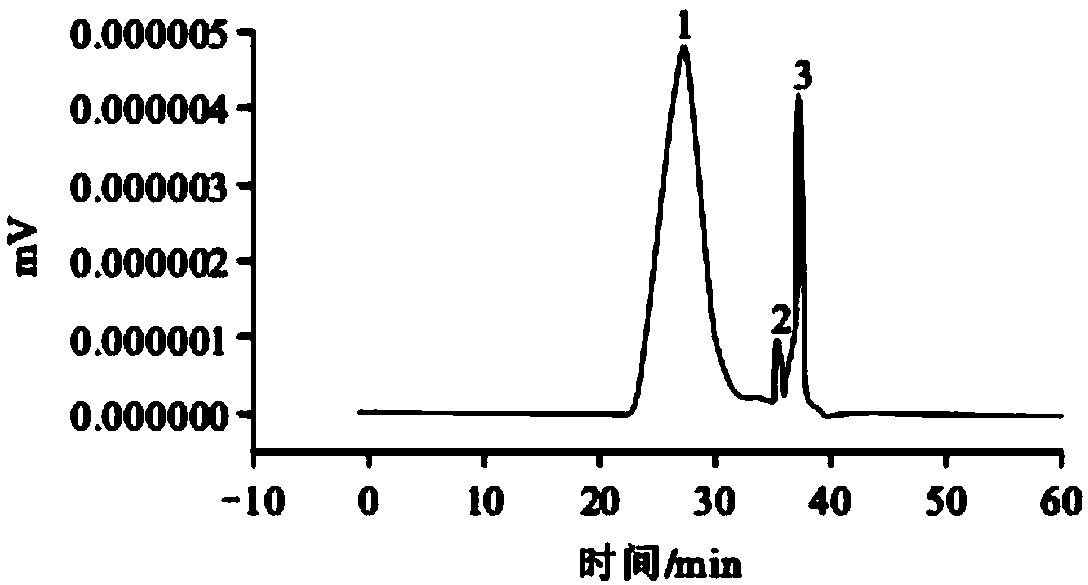
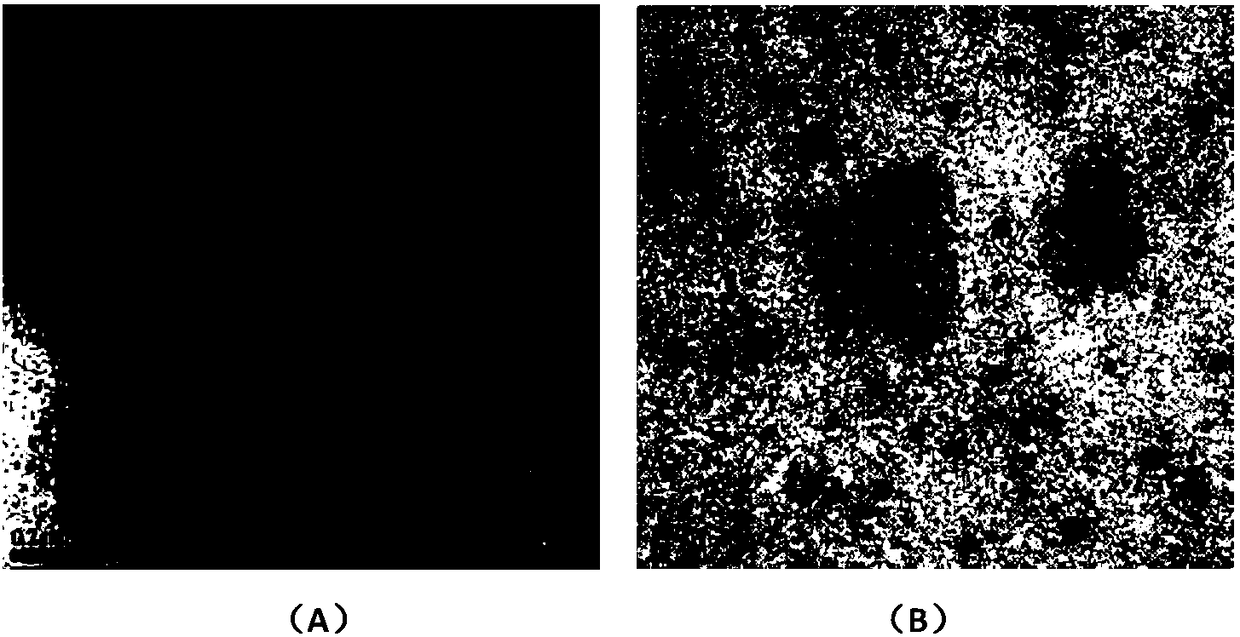
A compound immune adjuvant and its preparation method and application
A technology of compounding immune adjuvant and water for injection, which is applied in the field of medicine and biology to achieve the effects of enhancing immune response, enhancing immune protection effect and improving immune protection effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: (1) Preparation of Houttuynia cordata polysaccharide
[0034] Houttuynia cordata polysaccharide is prepared from dried Houttuynia cordata by the following process: raw material of dried houttuynia cordata → pulverizer crushing → hot water extraction → centrifugation, suction filtration → filtrate → vacuum concentration to 1 / of the original volume 8 Alcohol precipitation overnight → centrifugal separation, washing → vacuum drying → Houttuynia cordata polysaccharide, the specific process includes the following steps:
[0035] Step 1, taking dried Houttuynia cordata as raw material, using a pulverizer to pulverize it to 120 mesh to obtain Houttuynia cordata powder;
[0036] Step 2, mix Houttuynia cordata powder with warm water at 68°C in a ratio of 20:1 for hot water extraction, keep the extraction temperature at 68°C, stir and extract at a speed of 40rpm for 2.5 hours, and obtain Houttuynia cordata hot water immersion Extract;
[0037] Step 3: centrifuge the...
Embodiment 2
[0040] Embodiment 2: A compound immune adjuvant, consisting of the following components in weight percentage: Houttuynia cordata polysaccharide 2.5%, alanine 0.2%, ginkgo leaf flavonoids 1.4%, polyethylene castor oil 7%, Span80 7 %, polyethylene glycol 3.4%, squalene 8%, soybean oil for injection 32%, water for injection 38.5%.
[0041] The preparation method of described composite immune adjuvant comprises the steps:
[0042] Step 1, each raw material is weighed according to the weight percentage ratio;
[0043] Step 2, mix the weighed Houttuynia cordata polysaccharide and alanine with water for injection, stir until it is fully solvent, and obtain the water phase;
[0044] Step 3, mixing the weighed ginkgo leaf flavonoids with squalene and soybean oil for injection, and stirring evenly to obtain an oil phase;
[0045] Step 4, mix the weighed polyethylene castor oil, Span80 and polyethylene glycol, then add the oil phase prepared in step 3, and stir for 4 minutes at a speed...
Embodiment 3
[0047] Embodiment 3: A compound immune adjuvant, consisting of the following components in weight percentage: Houttuynia cordata polysaccharide 1.5%, alanine 0.55%, ginkgo leaf flavonoids 2.2%, polyethylene castor oil 5%, Span80 8 %, polyethylene glycol 5%, squalene 6%, soybean oil for injection 34%, water for injection 37.75%.
[0048] The preparation method of described composite immune adjuvant comprises the steps:
[0049] Step 1, each raw material is weighed according to the weight percentage ratio;
[0050] Step 2, mix the weighed Houttuynia cordata polysaccharide and alanine with water for injection, stir until it is fully solvent, and obtain the water phase;
[0051] Step 3, mixing the weighed ginkgo leaf flavonoids with squalene and soybean oil for injection, and stirring evenly to obtain an oil phase;
[0052] Step 4, mix the weighed polyethylene castor oil, Span80 and polyethylene glycol, then add the oil phase prepared in step 3, and stir for 5 minutes at a speed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com