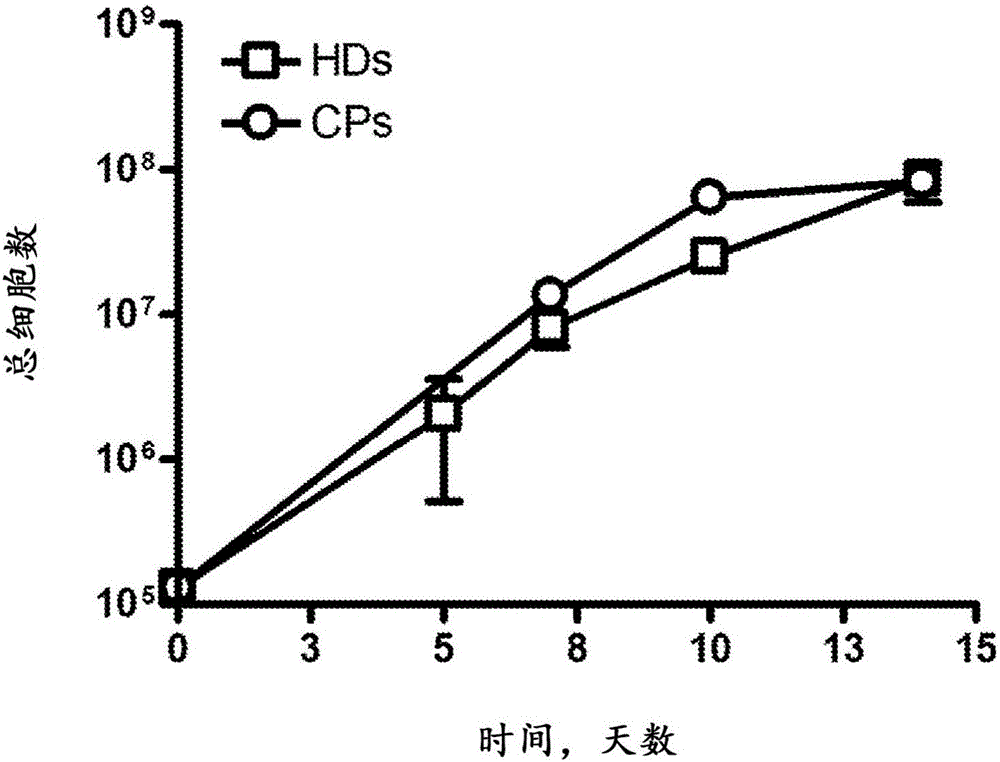
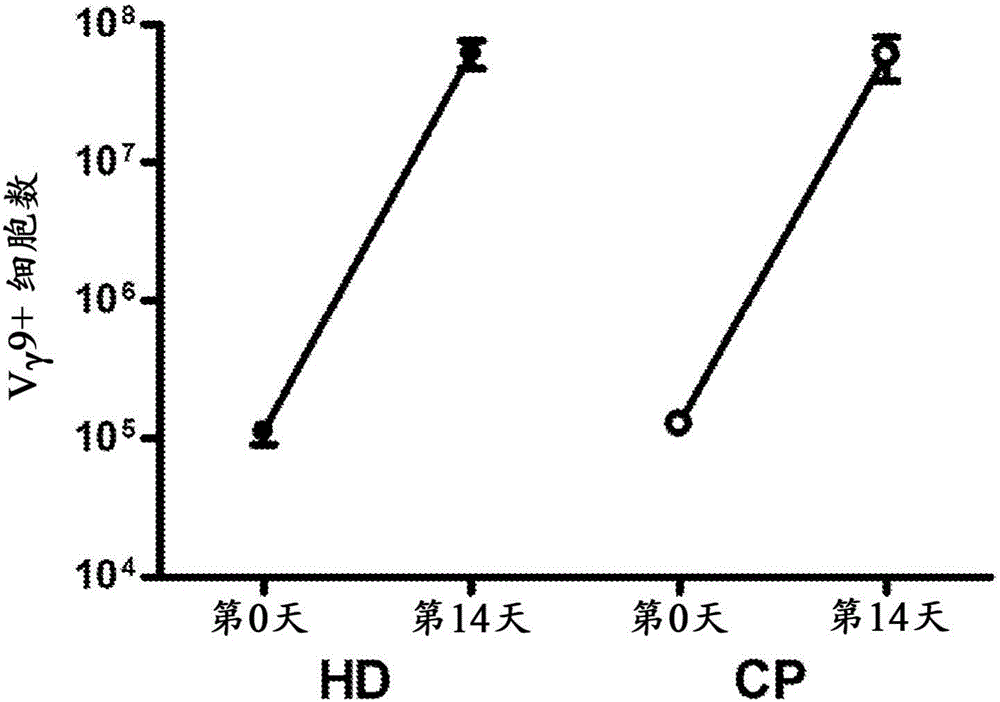
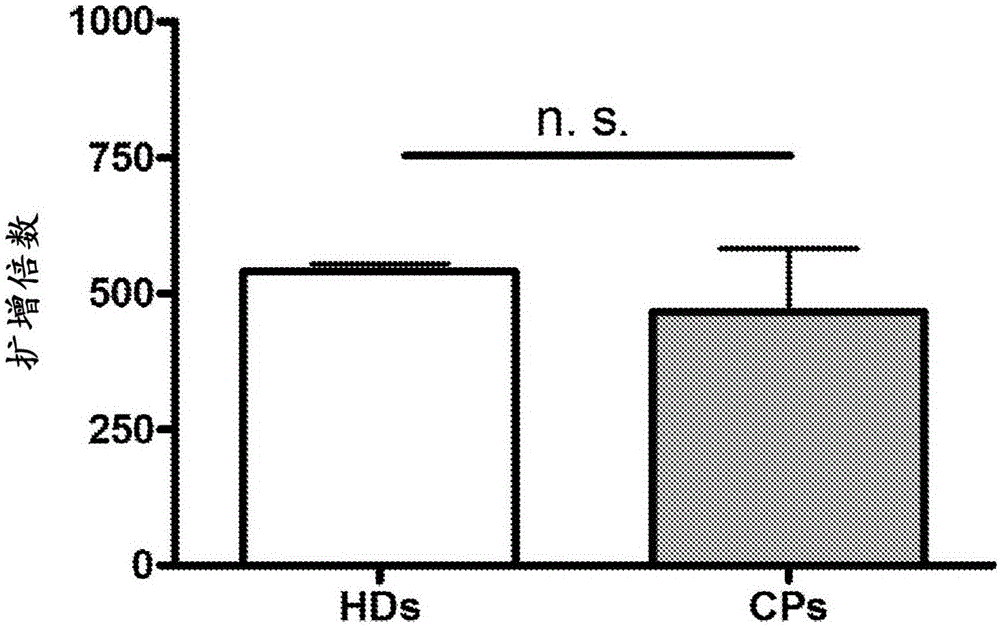
Expanding T cell populations using biphosphonates, anti CD 3 antibody and IL-2
A cell population, bisphosphonate technology, applied in autoimmune diseases and malignant diseases, γδT cells, treatment of infectious diseases, can solve the problems of γ9δ2T cell quantity and function inhibition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0179] Example 1 - Expansion of T cells derived from PBMC cells
[0180] Peripheral blood units of 100-500 ml were drawn from consenting individuals and subjected to Ficoll hypaque density centrifugation to obtain peripheral blood mononuclear cells (PBMC). Cells were subjected to the following expansion protocol for γ9δ2 T cells, which resulted in approximately 500-fold unexpected cell expansion in each stage relative to the initial amount:
[0181] (a) Phase I Expansion (PIE) - Cells were expanded at 0.5-1X10 6 Cells / ml were seeded in culture flasks in RPMI medium supplemented with 10% fetal calf serum, 2 mM L-glutamine and penicillin-streptomycin solution (100 μg / ml). Cultures were stimulated with 2 μM zoledronate and 100 international units (IU) / ml of recombinant human IL-2 (rhIL-2) and maintained for 14 days; replaced every 3 days with fresh medium with IL-2 Medium. After the first incubation period, γδ T cells were purified from the total cell population using immuno...
Embodiment 2
[0188] Example 2 - Activity of expanded T cells
[0189] γδ T cells cultured, expanded and isolated as detailed in Example 1 were tested for reactivity against GBM cell lines using an IFNγ secretion assay. γδ T cells were incubated with three GBM cell lines U251, U87 and T98G at a 5:1 effector to target ratio with or without zoledronate for 48 hours. After incubation, supernatants were analyzed for IFNγ secretion. PTE-derived γδT cells established some spontaneous IFNγ secretion in culture medium (Med), but significantly higher IFNγ secretion in response to GBM cell lines (p Figure 5 ).
[0190] Taken together, the results demonstrate that the two-stage expansion method provides high yields of viable and efficient γδ T cells.
[0191] Additional studies for testing the activity of expanded effector γδ T cells were performed as follows: tumor cell lines T98G, U87, U251 (glioblastoma multiforme) and Daudi (lymphoma) target cells were treated with 2.5 mM 5,6-Carboxyfluoresce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com