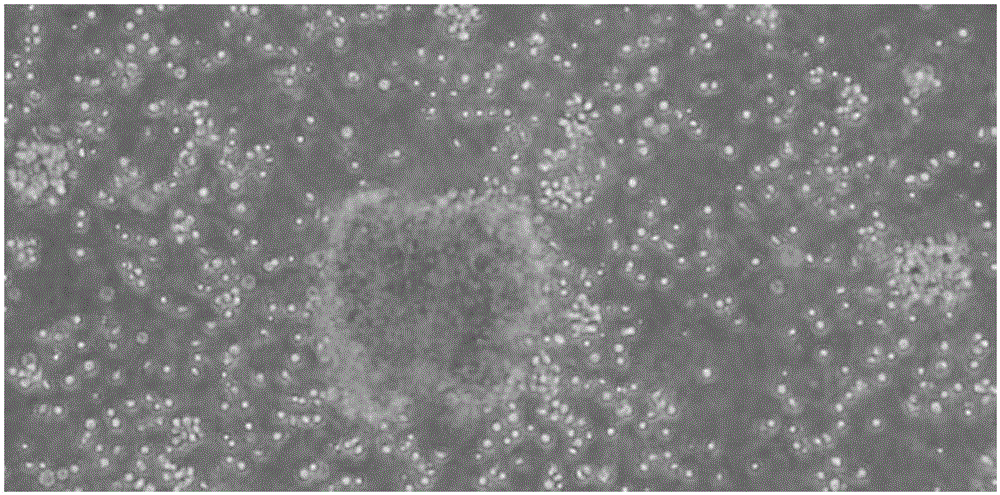
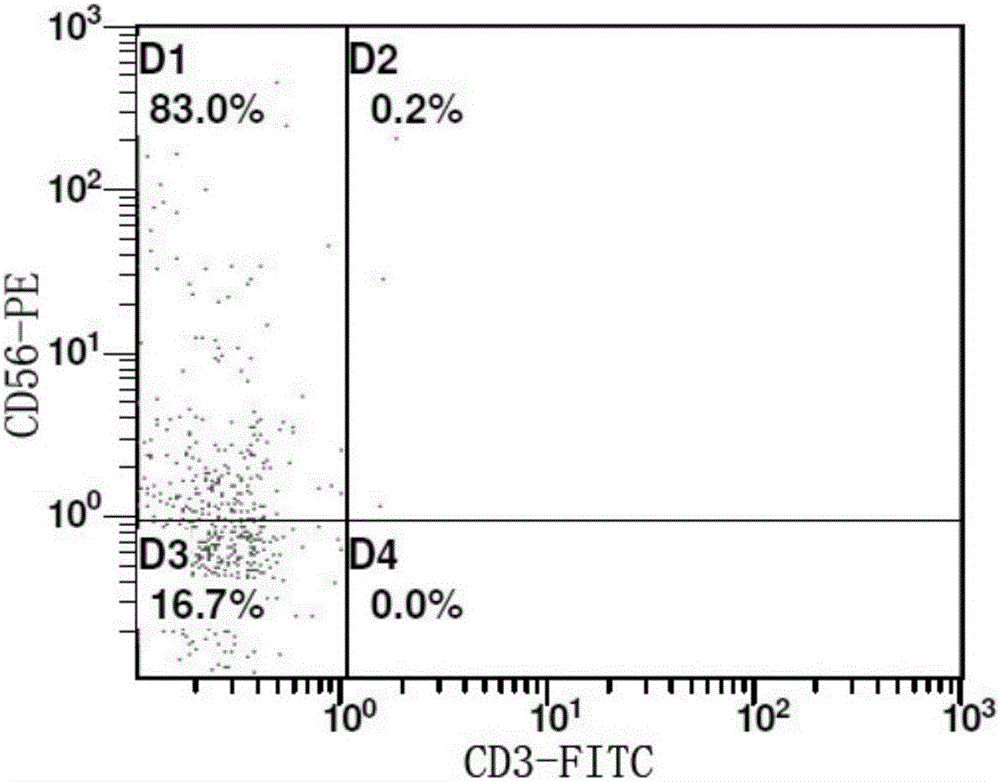
Method for in-vitro amplification of NK cells and NK cells obtained by same
A technology of NK cells and in vitro expansion, applied in the field of NK cells, can solve the problems of high preparation cost and difficult expansion of NK cells in vitro, and achieve the effect of reducing the amount of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1) Preparation of NK cell complete medium: immune cells containing FBS with a volume fraction of 6%, and IL-2 with a final concentration of 1000IU / ml, IL-12 with a final concentration of 50IU / ml, and IL-15 with a final concentration of 50IU / ml Culture medium;
[0058] 2) Prepare CD16Mab coating solution with NK cell complete medium: NK cell complete medium contains 8ug / ml CD16Mab;
[0059] 3) Add 5ml of CD16Mab coating solution to the T-175 cell culture flask, and coat for 24 hours;
[0060] 4) The PBMCs obtained from peripheral blood were separated according to 5*10 5 Inoculate the cells at a final concentration of / ml into the pre-coated cell culture flask, add NK cell complete medium until the culture system is 50ml, add IFN-r according to the final concentration of 1000IU / ml, and start the induction culture;
[0061] 5) On the fourth day of induction culture, add 50ml NK cell complete medium to the culture system to continue the culture;
[0062] 6) On the sixth ...
Embodiment 2
[0072] 1) Preparation of NK cell complete medium: immune cells containing FBS with a volume fraction of 5%, and IL-2 with a final concentration of 500IU / ml, IL-12 with a final concentration of 20IU / ml, and IL-15 with a final concentration of 20IU / ml Culture medium;
[0073] 2) Prepare CD16Mab coating solution with NK cell complete medium: NK cell complete medium contains 4ug / ml CD16Mab;
[0074] 3) Add 1ml of CD16Mab coating solution to the T-75 cell culture flask, and coat for 4 hours;
[0075] 4) The PBMCs obtained from peripheral blood were separated according to 3*10 5 Inoculate cells at a final concentration of 500IU / ml into cell culture flasks coated in advance, add NK cell complete medium to a culture system of 20ml, add IFN-r at a final concentration of 500IU / ml, and start induction culture;
[0076] 5) On the fourth day of induction culture, add 20ml NK cell complete medium to the culture system to continue the culture;
[0077] 6) On the seventh day of culture, ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com