Isoeugenol monooxygenase mutant and application thereof
A technology of isoeugenol and monooxygenase, applied in the biological field, can solve problems affecting industrial application and the like, and achieve the effect of high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Site-directed mutagenesis of isoeugenol monooxygenase site 120
[0058] For the technique of site-directed mutagenesis, please refer to the description of Ho et al. Gene. 1989, 77:51-33. The specific process is as follows:
[0059] In order to mutate Nsn (N) at position 120 in the parental amino acid sequence to Ile (I) to obtain mutant N 120I, the plasmid pET30a-ISO was used as a template to design primer pairs Nsn120-F and Nsn120-R (see Table 1) .
[0060] The F-NR fragment was amplified with the primer pair NdeI-F and N120-R, and the NF-R fragment was amplified with the primer pair Nsn120-F and XhoI-R. The amplification reaction conditions were: 25 μL Max DNA polymerase (TaKaRa, Japan), 2 μL pET21a-ISO, and 1.5 μL primer NdeI-F and 1.5 μL primer Nsn120-R (or, 1.5 μL primer Nsn120-F and 1.5 μL primer XhoI -R), adjust the reaction volume to 50 microliters with sterile water. The PCR amplification reaction program was: 98°C for 2 minutes, 30 cycles: 98°C ...
Embodiment 2
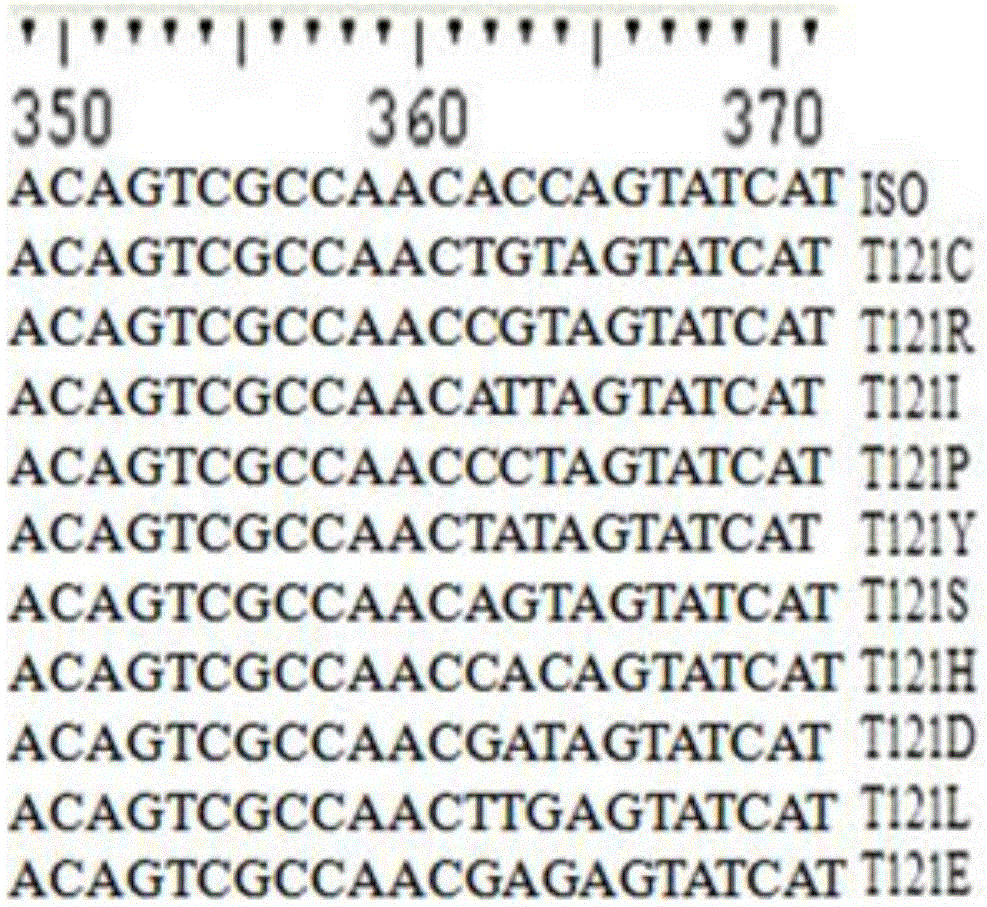
[0063] Example 2: Site-directed mutagenesis of isoeugenol monooxygenase site 121
[0064] The technique of site-directed mutagenesis can refer to the description of Ho et al. Gene. 1989, 77:51-33. The specific process is as follows:
[0065] In order to mutate Thr(T) at position 121 in the parental amino acid sequence to Pro(P) to obtain mutant T120P, the plasmid pET30a-ISO was used as a template to design primer pairs Thr121-F and Thr121-R (see Table 1).
[0066]The F-TR fragment was amplified with the primer pair NdeI-F and Thr121-R, and the TF-R fragment was amplified with the primer pair Thr121-F and XhoI-R. The amplification reaction conditions were: 25 μL Max DNA polymerase (TaKaRa, Japan), 2 μL pET21a-ISO, and 1.5 μL primer NdeI-F and 1.5 μL primer Thr121-R (or, 1.5 μL primer Thr121-F and 1.5 μL primer XhoI -R), adjust the reaction volume to 50 microliters with sterile water. The PCR amplification reaction program was: 98°C for 2 minutes, 30 cycles: 98°C for 10 secon...
Embodiment 3
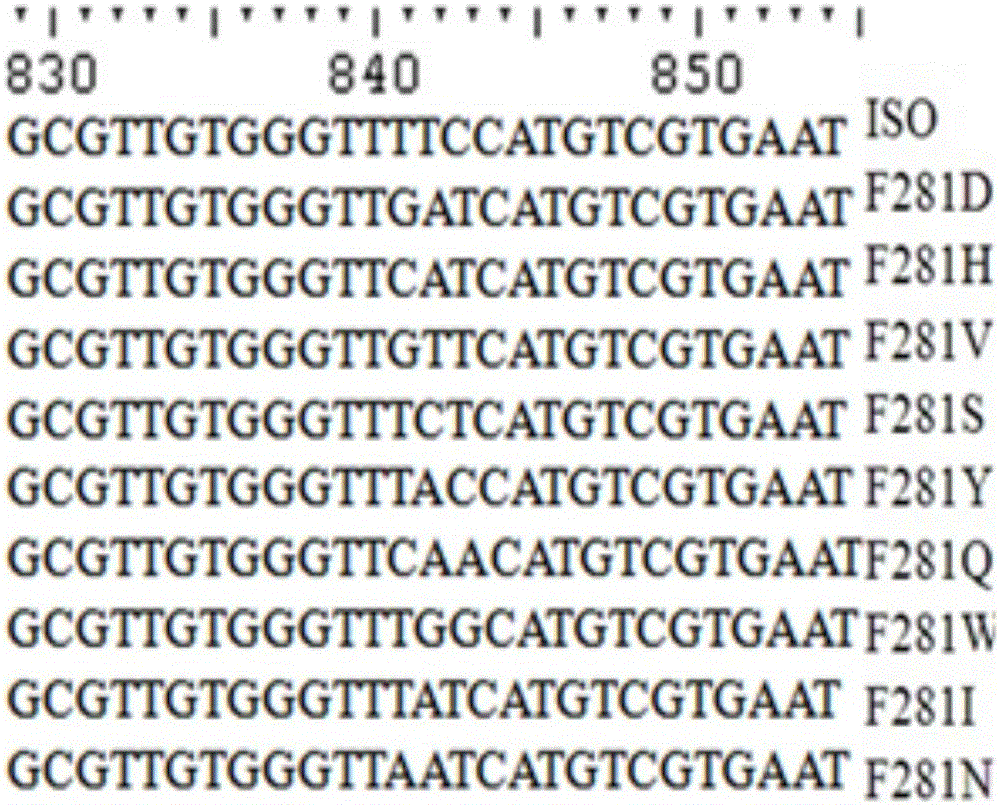
[0069] Example 3: Site-directed mutation of isoeugenol monooxygenase site 281
[0070] The technique of site-directed mutagenesis can refer to the description of Ho et al. Gene. 1989, 77:51-33. The specific process is as follows:
[0071] In order to mutate Phe(F) at position 281 in the parental amino acid sequence to Gln(Q) to obtain mutant F281Q, the plasmid pET30a-ISO was used as a template to design primer pairs Phe281-F and Phe281-R (see Table 1).
[0072] The primer pair NdeI-F and Phe281-R was used to amplify the F-FR fragment, and the primer pair Phe281-F and XhoI-R was used to amplify the FF-R fragment. The amplification reaction conditions were: 25 μL Max DNA polymerase (TaKaRa, Japan), 2 μL pET21a-ISO, and 1.5 μL primer NdeI-F and 1.5 μL primer Phe281-R (or, 1.5 μL primer Phe281-F and 1.5 μL primer XhoI -R), adjust the reaction volume to 50 microliters with sterile water. The PCR amplification reaction program was: 98°C for 2 minutes, 30 cycles: 98°C for 10 secon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com