Mycobacterium tuberculosis specificity CTL epitope peptides and application thereof
A technology of Mycobacterium tuberculosis and epitope peptides, which is applied in the fields of molecular biology and immunology, can solve the problems of unclear characteristics of tuberculosis vaccines, no MHC typing of peptides, affinity and stability to be identified, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Preparation of HLA-A2-restricted CTL epitope polypeptide of Mycobacterium tuberculosis-specific protein Rv2629
[0036] 1. Prediction of HLA-A2-restricted CTL epitopes of Mycobacterium tuberculosis-specific protein Rv2629 using bioinformatics methods
[0037] Mycobacterium tuberculosis specific protein Rv2629, its amino acid composition is shown in SEQ ID NO.13.
[0038] SEQ ID NO.13:
[0039]MRSERLRWLVAAEGPFASVYFDDSHDTLDAVERREATWRDVRKHLESRDAKQELIDSLEEAVRDSRPAVGQRGRALIATGEQVLVNEHLIGPPPATVIRLSDYPYVVPLIDLEMRRPTYVFAAVDHTGADVKLYQGATISSTKIDGVGYPVHKPVTAGWNGYGDFQHTTEEAIRMNCRAVADHLTRLVDAADPEVVFVSGEVRSRTDLLSTLPQRVAVRVSQLHAGPRKSALDEEEIWDLTSAEFTRRRYAEITNVAQQFEAEIGRGSGLAAQGLAEVCAALRDGDVDTLIVGELGEATVVTGKARTTVARDADMLSELGEPVDRVARADEALPFAAIAVGAALVRDDNRIAPLDGVGALLRYAATNRLGSHRS
[0040] 1. SYFPEITHI supermotif method for remote prediction of CTL epitopes:
[0041] Using SYFPEITHI online software (Ver.1.0) to predict the CTL epitope consisting of 9 amino acid residues;
[00...
experiment example 1
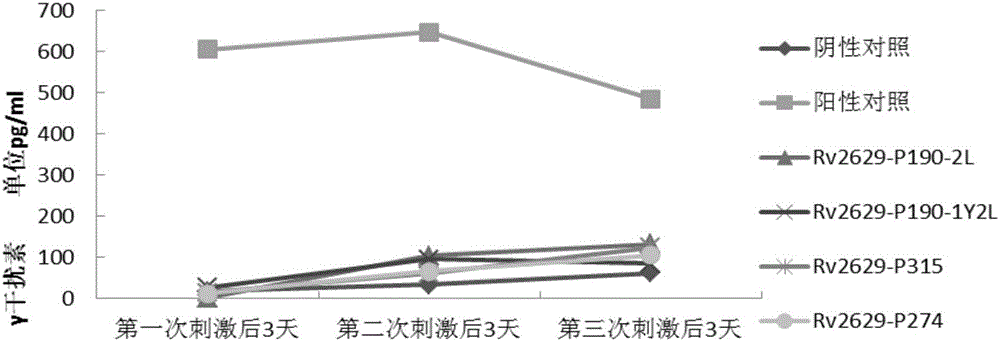
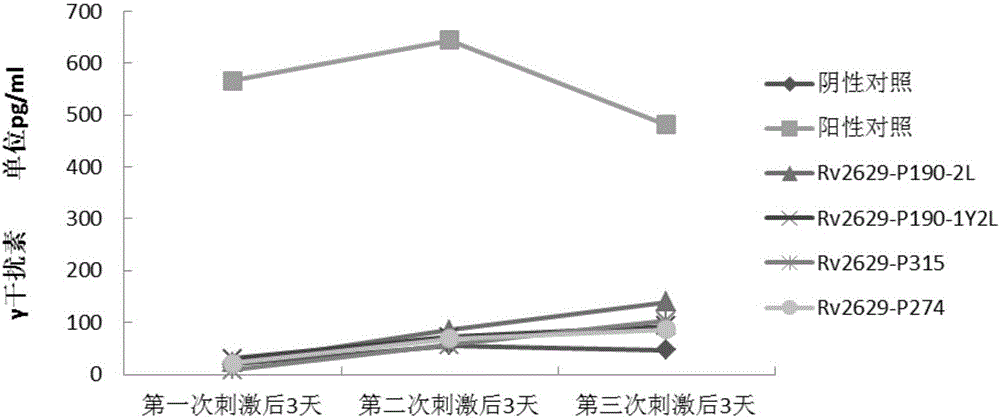
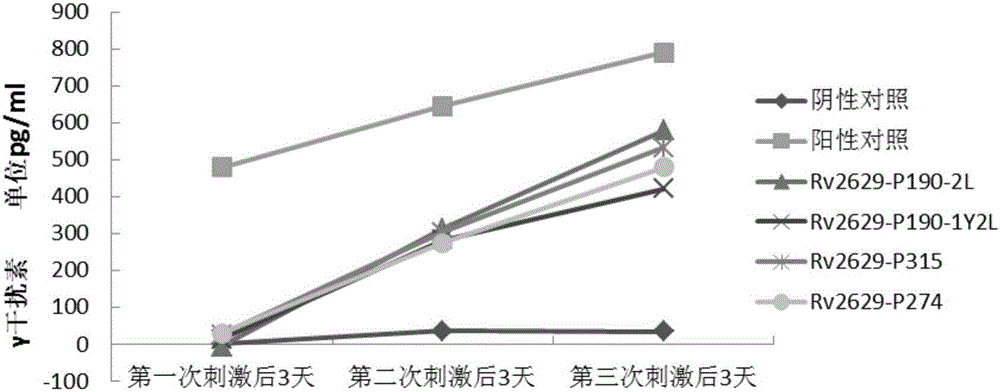
[0084] Experimental example 1: In vitro immune activity detection of epitope polypeptides
[0085] 1. Materials and methods
[0086] 1. Experimental materials
[0087] 1.1 Experimental cell lines
[0088] The T2 cell line was donated by the Department of Immunology, Third Military Medical University, and cultured in RPMI1640 medium containing 10% fetal bovine serum in a 37°C, 5% carbon dioxide incubator.
[0089] 1.2 Peripheral blood samples
[0090] Peripheral blood samples were provided by the Army Tuberculosis Research Institute and were divided into normal control group, LTBI group and tuberculosis group. The HLA phenotype analysis of peripheral blood was detected by flow cytometry in the organ transplantation laboratory, all of which were positive for HLA-A2.
[0091] 1.3 Main reagents
[0092] RPMI1640 medium: product of Gibco, USA;
[0093] IMDM medium: product of Gibco, USA;
[0094] Fetal bovine serum: product of Gibco, USA;
[0095] Glutamine: product of Sigma...
experiment example 2
[0164] Experimental example 2: Lactate dehydrogenase method (Lactate dehydrogenase, LDH) detection of specific CTL cytotoxic killing experiment
[0165] 1. Materials and methods
[0166] 1. Experimental materials
[0167] 1.1 Experimental cell lines
[0168] The T2 cell line was donated by the Department of Immunology, Third Military Medical University of the Chinese People's Liberation Army, and cultured in RPMI1640 medium containing 10% fetal bovine serum in a 37°C, 5% carbon dioxide incubator.
[0169] 1.2 Peripheral blood samples
[0170] Peripheral blood samples were provided by the Army Tuberculosis Research Institute and were divided into normal control group, LTBI group and tuberculosis group. The HLA phenotype analysis of peripheral blood was detected by flow cytometry in the organ transplantation laboratory, all of which were positive for HLA-A2.
[0171] 1.3 Main reagents
[0172] RPMI1640 medium: product of Gibco, USA;
[0173] IMDM medium: product of Gibco, U...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com