Cis-platinum complex and application thereof
A complex and fluorescence technology, applied in the field of biochemistry, can solve the problems of unstable fluorescence intensity, low labeling efficiency, long labeling time, etc., and achieve the effect of remarkable labeling effect, excellent labeling effect and fast labeling speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
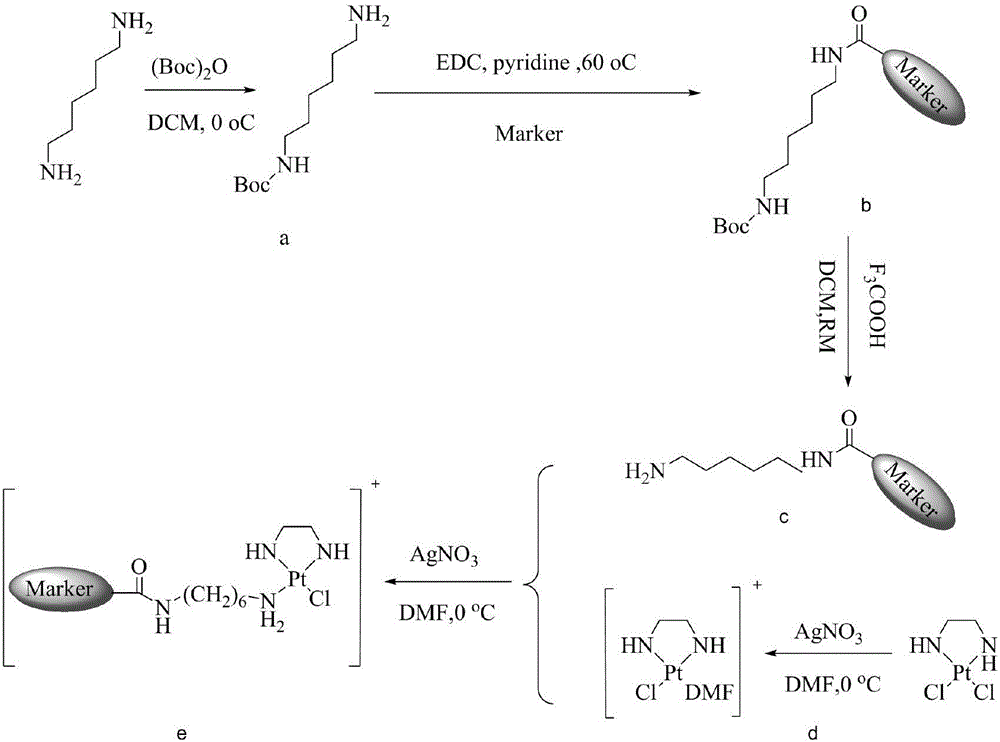
[0035] Synthesis of the cisplatin fluorescent complex of the present invention when n=6
[0036] Such as figure 2 Shown synthetic route, concrete steps are as follows:
[0037] (1) with 1,6-diaminohexane as the starting reactant; first (Boc) 2 O (12.3g, 0.056mol) was dissolved in 100mL of dichloromethane, then slowly added dropwise into 150mL of dichloromethane solution dissolved in 1,6-diaminohexane (33g, 0.28mol), and reacted at 0°C for 4 hours , and then reacted at room temperature for 20 hours; after the reaction was complete, wash the reaction solution with 4×100mL saturated brine, separate the liquids, dry the organic phase with anhydrous sodium sulfate, and evaporate the solvent under reduced pressure to obtain 14.3g colorless oily liquid, namely N-Boc-1,6-diaminohexane (N-Boc-1,6-diaminohexane, intermediate a) was directly carried out to the next reaction without purification.
[0038] (2) Dissolve [Pt(en)Cl2] (500mg, 1.53mmol) in 50ml DMF, then add AgNO 3 (248mg,...
Embodiment 2
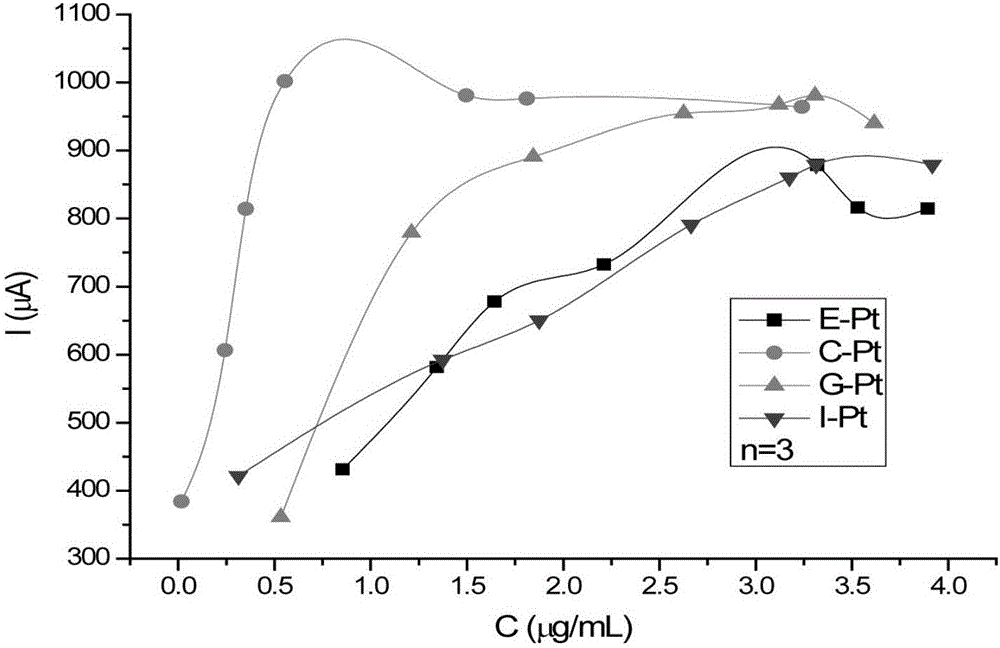
[0041] In this experiment, platinum was labeled with rhodamine, DEAC, gloxalic acid, FITC, TRITC, Alexa fluor 594 and other fluoresceins, and the fluorescence intensity was measured. The results indicated that the cisplatin-mediated complex could combine well with the above fluorescein and emit fluorescence. (n=3, 6, 9 for the cisplatin fluorescent complexes used in this experiment). The marker-Pt coordination compound was dissolved in a 0.1 M tris buffer solution, pH = 7.1, and the fluorescence intensity was measured at room temperature. The relationship between the fluorescence intensity and the complex concentration of organic cisplatin fluorescent compounds (carbon chain lengths are respectively n=3, n=6, n=9) is as follows Figure 3-5 shown.
[0042] We also used organic cisplatin fluorescent complexes to bind biotin, and used avidin and Cy5-labeled fluorescent antibodies to label to form biotin-avidin-fluorescent antibody complexes, and the fluorescent signal was signi...
Embodiment 3
[0044] Experiments of preparing nucleic acid and protein probes using cisplatin fluorescent complexes of the present invention
[0045] 1. Nucleotide labeling method
[0046] Mix 100ug of purified target nucleotide clones with 2ug of purified platinum-biotin-fluorescein (FITC, Cy5) markers (n=6 in the cisplatin fluorescent complex used in this experiment), and react at 80°C for about 40min (the reaction time can be appropriately adjusted according to the type of target molecule, and the RNA reaction time can be shortened to about 20min), suck out the mixture of labeled samples, and pass through the above-mentioned cellulose acetate liquid phase separation column to obtain the purified fluoresceinized nucleotide sequence .
[0047] 2. Protein Molecular Probe Labeling Method
[0048] Mix 100ug of protein (antibody IgG used in this experiment) with 1ug of purified platinum-biotin-fluorescein (FITC, Cy5) label (n=6 in this experiment using cisplatin fluorescent complex), 37°C A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com