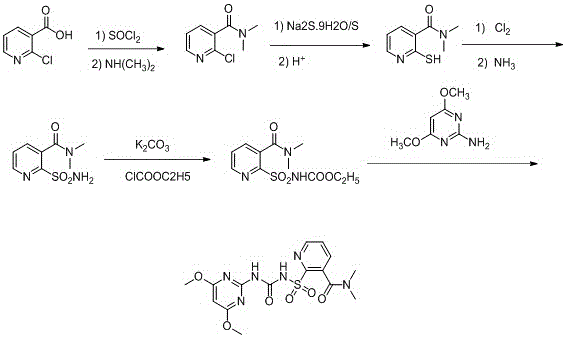
Synthesis process of nicosulfuron original medicine
The technology of nicosulfuron-methyl technical and rimsulfuron-methyl technical application is applied in the field of pesticide synthesis, which can solve the problems of high equipment requirements, environmental pollution, excessive phosgene, etc., and achieves improved synthesis quality and yield, and good atom economy. , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1) In a 250ml flask, add 31.5g (0.2mol) of 2-chloronicotinic acid, then add 81.5g (1.46mol) of thionyl chloride, heat to reflux, keep the reaction for 4 hours, the HPLC detection content is 98.9%, and cool down , and the excess thionyl chloride was distilled off under reduced pressure.
[0021] 2) Add about 100ml of dichloromethane, completely dissolve the solid, cool to 10°C, then add 79g (0.513mol) of 33% dimethylamine aqueous solution dropwise at a constant temperature for 2 hours, stir for 4 hours, and the HPLC detection content is 92 %.
[0022] 3) Stop the reaction, add concentrated hydrochloric acid, adjust the pH to about 3, let stand to separate the liquid, filter, and concentrate the filtrate under reduced pressure to obtain a solid.
[0023] 4) Add about 115ml of 48g (0.1mol) Na 2 S.9H 2 O and 25.6g (0.2mol) of elemental sulfur in aqueous solution were heated to reflux for 20 hours.
[0024] 5) Stop heating, cool to room temperature, add concentrated hydr...
Embodiment 2
[0030] 1) Add 0.22mol of 2-chloronicotinic acid, then add 1.3mol of thionyl chloride, heat to reflux, keep warm for 4 hours, the HPLC detection content is 99.2%, lower the temperature, and remove excess thionyl chloride by vacuum distillation;
[0031] 2) Add 100ml of dichloromethane, dissolve the solid completely, cool to 8°C, then add 0.52mol of 33% dimethylamine aqueous solution dropwise at a constant temperature for 2.2 hours, stir for 3.5 hours, and the HPLC detection content is 92.4%;
[0032] 3) Stop the reaction, add concentrated hydrochloric acid, adjust the pH to 3, let stand to separate the liquid, filter, and concentrate the filtrate under reduced pressure to obtain a solid;
[0033] 4) Add 110ml of 0.09mol Na 2 S.9H 2 O and 0.2mol elemental sulfur aqueous solution, heated to reflux for 22 hours;
[0034] 5) Stop heating, cool to room temperature, add concentrated hydrochloric acid dropwise, adjust the pH value to 2.8, raise the temperature to 75°C, stir for 35 m...
Embodiment 3
[0040] 1) Add 0.22mol of 2-chloronicotinic acid, then add 1.5mol of thionyl chloride, heat to reflux, keep warm for 3.8 hours, the HPLC detection content is 99%, lower the temperature, and remove excess thionyl chloride by vacuum distillation;
[0041] 2) Add 100ml of dichloromethane, dissolve the solid completely, cool to 9°C, then add 0.498mol of 33% dimethylamine aqueous solution dropwise at a constant temperature for 2.3 hours, stir for 5 hours, and the HPLC detection content is 92.6%;
[0042] 3) Stop the reaction, add concentrated hydrochloric acid, adjust the pH to 3.2, let stand to separate the liquid, filter, and concentrate the filtrate under reduced pressure to obtain a solid;
[0043] 4) Add 125ml of 0.12mol Na 2 S.9H 2 O and 0.24mol elemental sulfur aqueous solution were heated to reflux for 20 hours;
[0044] 5) Stop heating, cool to room temperature, add concentrated hydrochloric acid dropwise, adjust the pH value to 3.2, raise the temperature to 75°C, stir fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com