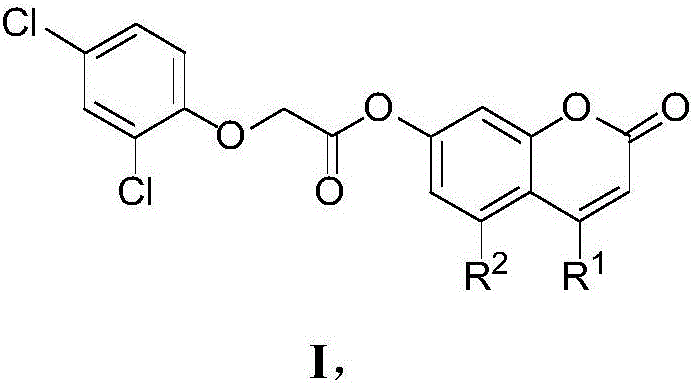
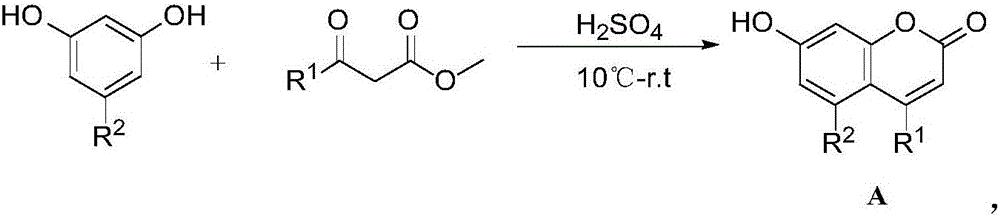
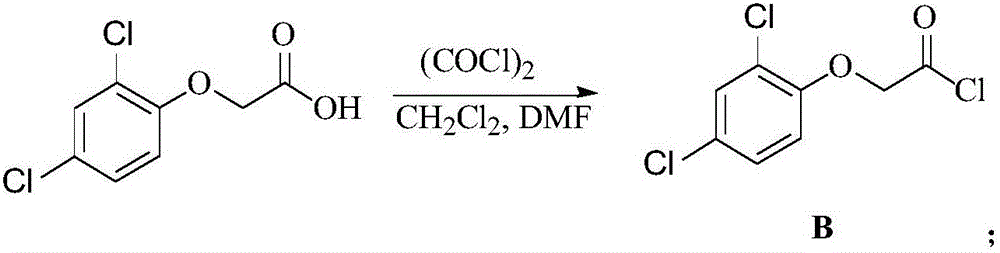7-aryloxyacetyl coumarin compound and application thereof in pesticides
An aryloxyacetoxy, coumarin technology, applied in the application, biocide, organic chemistry and other directions, to achieve the effect of improving herbicidal activity, high herbicidal activity, and high control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of 7-hydroxyl-4,5-dimethylcoumarin (A1):
[0032] The structural formula is:
[0033] 0.62g (5mmol) of 5-methylresorcinol and 8.0mL of concentrated sulfuric acid were stirred to dissolve, and 0.65mL (5mmol) of ethyl acetoacetate was slowly added dropwise under cooling in an ice-water bath; Reaction at room temperature for 24h. Pour into a large amount of ice water under vigorous stirring, a precipitate is formed, filter with suction, and recrystallize the filter cake with absolute ethanol to obtain 0.74g of 7-hydroxy-4,5-dimethylcoumarin (A1) as a white powder. Rate 78.2%, mp: 251~253℃; 1 H NMR (400MHz, DMSO-d 6 )δ:2.28(s,3H,CH 3 ),2.54(s,3H,CH 3 ),5.93~6.11(m,1H,H-6),6.52~6.69(m,1H,H-8),10.53(s,1H,OH); 13 C NMR (400MHz, DMSO-d 6 )δ: 160.3, 156.9, 155.3, 155.0, 143.2, 112.4, 112.3, 108.2, 101.0, 23.9, 21.6. Anal. Calcd for C 11 h 10 o 3 : C 69.46, H 5.30; found C 69.58, H 5.07.
Embodiment 2
[0034] Embodiment 2: Preparation of 7-hydroxyl-4-chloromethyl-5-methylcoumarin (A2):
[0035] The structural formula is:
[0036] 0.62g (5mmol) of 5-methylresorcinol and 8.0mL of concentrated sulfuric acid were stirred to dissolve, and 0.68mL (5mmol) of ethyl 4-chloromethylacetoacetate was slowly added dropwise under cooling in an ice-water bath. Reaction under cooling for 1h; reaction at room temperature for 24h. Pour into a large amount of ice water under vigorous stirring, a precipitate is formed, filter with suction, and recrystallize the filter cake with absolute ethanol to obtain 0.90 g of 7-hydroxy-4-chloromethyl-5-methylcoumarin (A2), white Powder, yield 80.3%, mp: 275~277℃; 1 H NMR (400MHz, DMSO-d 6 )δ:2.29(s,3H,CH 3 ),5.08(s,2H,CH 2 ),6.41(s,1H,H-3),6.55~6.62(m,1H,H-6),6.64~6.70(m,1H,H-8),10.92(s,1H,OH); 13 C NMR (400MHz, DMSO-d 6 )δ: 160.2, 156.1, 155.3, 152.2, 143.9, 112.6, 112.5, 108.4, 104.8, 45.5, 21.6. Anal. Calcd for C 11 h 9 ClO 3 : C58.81, H 4.04...
Embodiment 3
[0037] Embodiment 3: Preparation of 7-hydroxyl-4-trifluoromethyl-5-methylcoumarin (A3):
[0038] The structural formula is:
[0039] 0.62g (5mmol) of 5-methylresorcinol and 8.0mL of concentrated sulfuric acid were stirred to dissolve, and 0.73mL (5mmol) of ethyl 4,4,4-trifluoroacetoacetate was slowly added dropwise under cooling in an ice-water bath. , Reacted for 1h under cooling in an ice-water bath; reacted for 24h at room temperature. Poured into a large amount of ice water under vigorous stirring, a precipitate formed, suction filtered, and the filter cake was recrystallized with absolute ethanol to obtain 0.95 g of 7-hydroxy-4-trifluoromethyl-5-methylcoumarin (A3). White powder, yield 77.9%, mp: 255~256℃; 1 H NMR (400MHz, DMSO-d 6 )δ:2.51(s,3H,CH 3), 4.16(s, 1H, OH), 6.64~6.71(m, 1H, H-3), 6.73~6.81(m, 2H, H-6, H-8); 13 C NMR (400MHz, DMSO-d 6 )δ: 161.3, 158.9, 158.0, 145.1, 137.3, 118.6, 113.0, 105.8, 102.4, 22.3, 21.6. Anal. Calcd for C 11 h 7 f 3 o 3 : C 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com