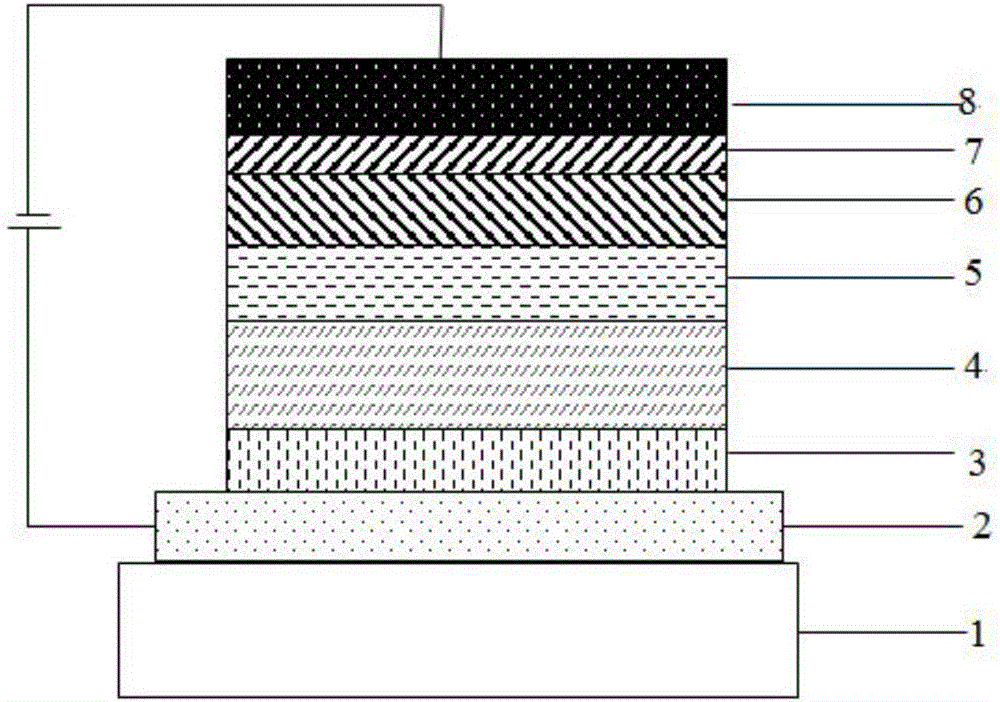
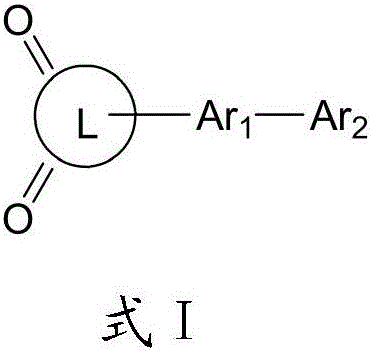
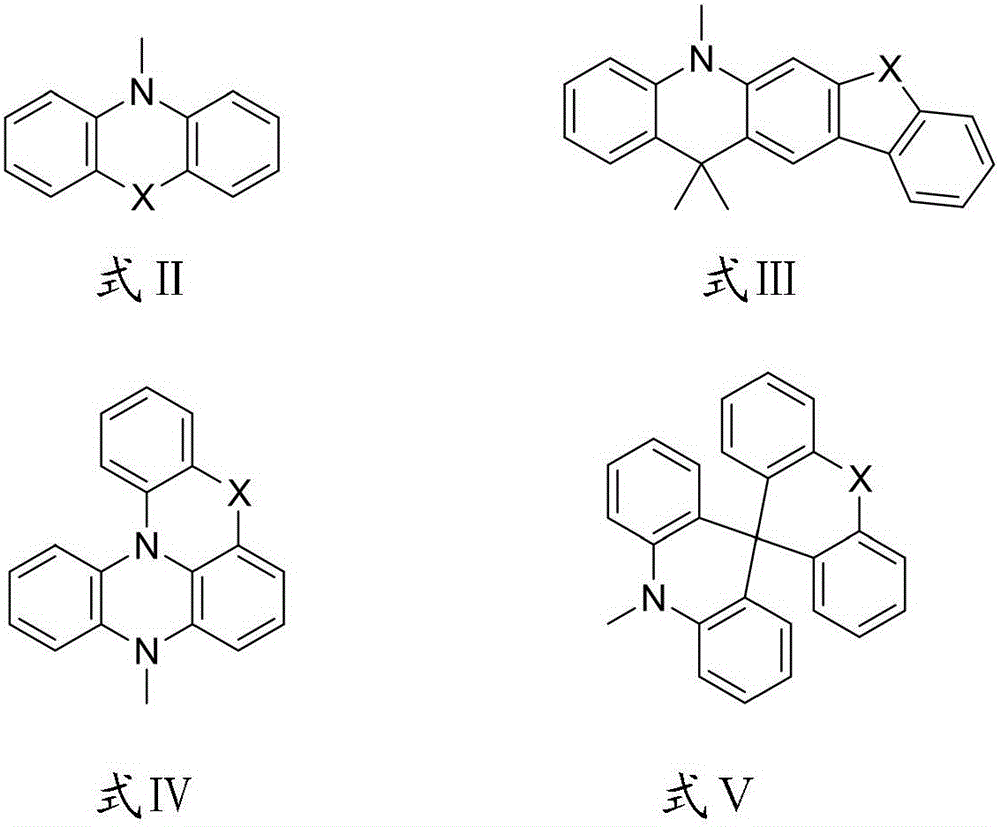
Thermally-activated delayed fluorescence OLED material taking cyclic diketone as core and application of OLED material
A heat-activated delayed, cyclic diketone technology, applied in the direction of luminescent materials, electrical components, circuits, etc., can solve the problems of not being able to meet high-efficiency organic light-emitting diodes, limit the development of efficient delayed fluorescent materials, reduce luminous efficiency, etc., and achieve good applications effect, good performance, and long device life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the preparation of compound C1
[0040]
[0041] In a 500mL three-necked flask, add 2-(4-bromophenyl)cyclohexane-1,3-dione (2.67g, 0.01mol), 5-phenyl-5,10-dihydrophenazine (2.58g , 0.01mol), sodium tert-butoxide (2.88g, 0.03mol), xylene (300mL), palladium acetate (0.074g), Xantphos (0.348g), under N2 protection, the temperature was raised to reflux, the reaction was kept for 12h, and the At room temperature, add 150mL deionized water to the reaction bottle, stir for 5min, separate the liquid, wash the organic phase twice with 200mL deionized water, collect the organic phase, dry with anhydrous Na2SO4, filter, remove the solvent, and purify the crude product by silica gel column chromatography , the eluent is toluene:petroleum ether=1:2, to obtain compound C1, 2.85 g of light yellow solid, yield 64.2%.
[0042] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 30 h 24 N 2 o 2 , the theoretical value is 444.1838, a...
Embodiment 2
[0043] Embodiment 2: the preparation of compound C4
[0044]
[0045]The preparation method of compound C4 is the same as that of Example 1, except that raw material A1 is used to replace 5-phenyl-5,10-dihydrophenazine in Example 1 to obtain compound C4, 3.23 g of light yellow solid, yield 66.7 %.
[0046] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 33 h 28 N 2 o 2 , the theoretical value is 484.2151, and the test value is 484.2331. Elemental analysis (C 33 h 28 N 2 o 2 ), theoretical value C: 81.79, H: 5.83, N: 5.78, O: 6.60, measured value C: 81.78, H: 5.84, N: 5.77, O: 6.61.
Embodiment 3
[0047] Embodiment 3: the preparation of compound C7
[0048]
[0049] The preparation method of compound C7 is the same as that of Example 1, except that raw material A2 is used to replace 5-phenyl-5,10-dihydrophenazine in Example 1 to obtain compound C7, 3.06 g of light yellow solid, yield 57.3 %.
[0050] High resolution mass spectrometry, ESI source, positive ion mode, molecular formula C 37 h 27 NO 3 , theoretical value 533.1991, test value 533.1860. Elemental analysis (C 37 h 27 NO 3 ), theoretical value C: 83.28, H: 5.10, N: 2.62, O: 8.99, measured value C: 83.30, H: 5.12, N: 2.61, O: 8.97.
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com