A kind of ethidium bromide derivative and its preparation and application in antitumor
A technology of ethidium bromide and derivatives, which is applied in the field of medicine, can solve the problems of tumor treatment failure, poor selectivity, and inability to effectively reach the action target, so as to reduce the level of reduced glutathione and increase the level of oxidative free radicals. Level and effect of large medical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
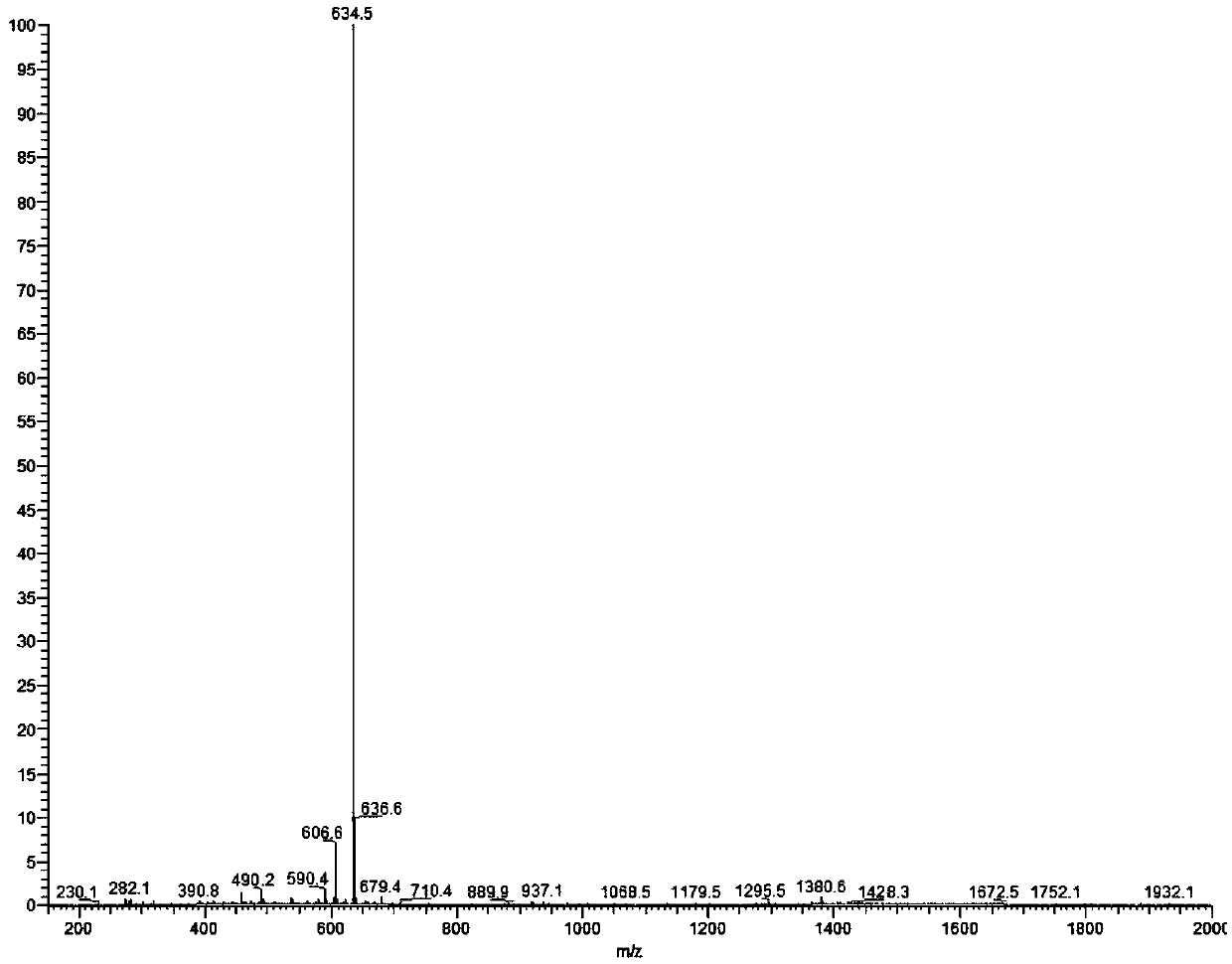
[0037] Synthesis of Antitumor Drug Compound 1:
[0038]
[0039] (1) EB (230 mg) was dissolved in 50 mL of a mixed solvent of trifluoroacetic acid / acetonitrile / dichloromethane with a volume ratio of 0.01:1:4. The mixture was protected with nitrogen and stirred at 0°C for 10 minutes.
[0040] (2) Sodium nitrite (150 mg) was added to the mixture obtained in step (1), and the mixture was kept at 0°C and stirred for 5 minutes.
[0041] (3) Add sulfamic acid (200mg) into the reaction solution obtained in step (2), and keep stirring at 0°C for 10 minutes.
[0042] (4) Dissolve N,N-diethylaniline (200 μL) in 2 mL of trifluoroacetic acid / acetonitrile / dichloromethane mixed solvent with a volume ratio of 0.01:1:4, and add it to the reaction solution obtained in step (3) , kept stirring at 0°C for 60 minutes.
[0043] (5) After the reaction, the reaction solution obtained in step (4) was diluted with dichloromethane, washed with water three times, and the organic phase was collected...
Embodiment 2
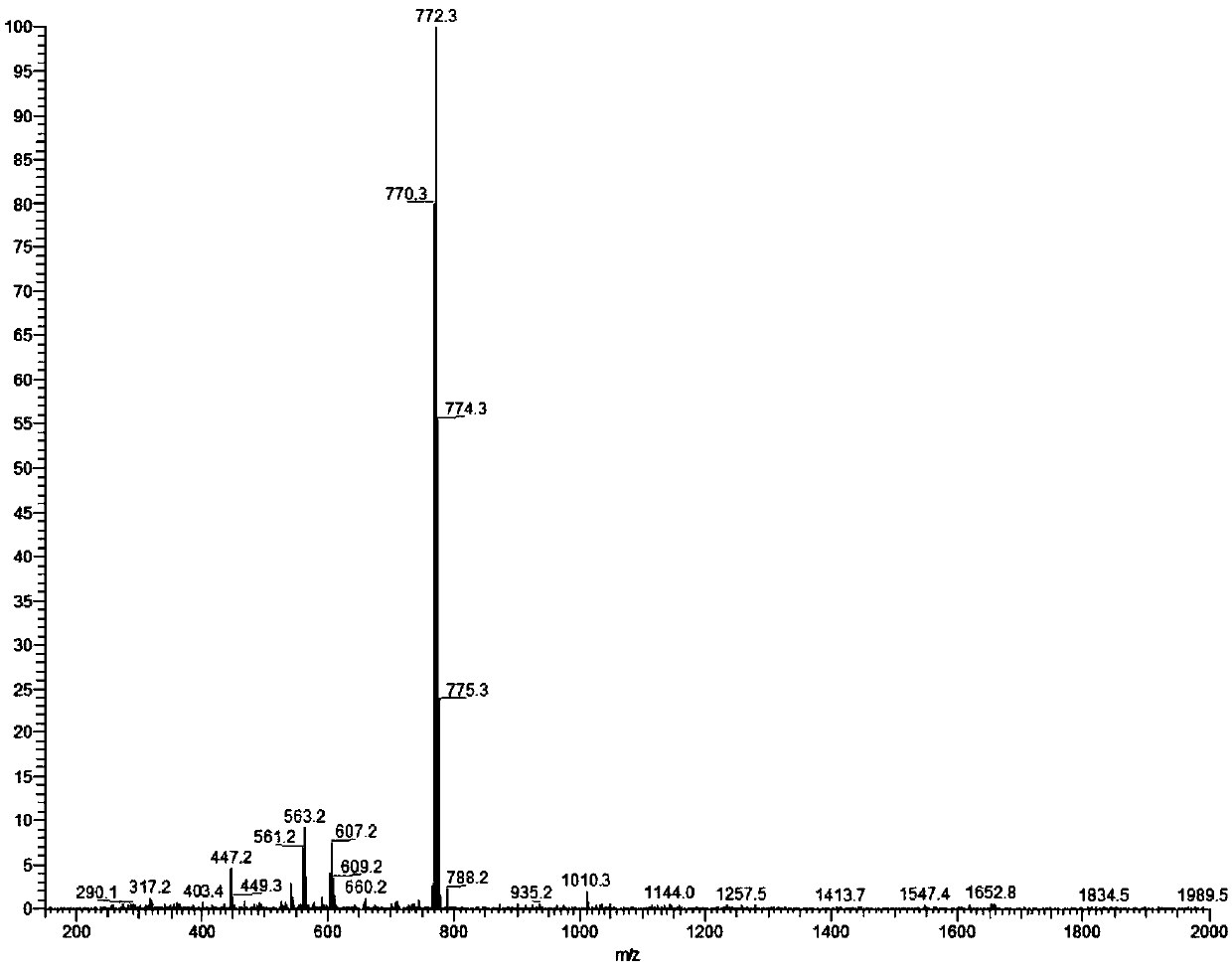
[0048] Synthesis of antitumor drug compound 2:
[0049]
[0050] (1) EB (230 mg) was dissolved in 50 mL of a mixed solvent of trifluoroacetic acid / acetonitrile / dichloromethane with a volume ratio of 0.01:1:4. The mixture was protected with nitrogen and stirred at 0°C for 5 minutes.
[0051] (2) Sodium nitrite (150 mg) was added to the mixture obtained in step (1), and the mixture was kept at 0° C. and stirred for 10 minutes.
[0052] (3) Add sulfamic acid (200mg) into the reaction solution obtained in step (2), keep stirring at 0°C for 5 minutes.
[0053](4) Dissolve N,N-bis(2-chloroethyl)aniline (200 μL) in 2 mL of trifluoroacetic acid / acetonitrile / dichloromethane mixed solvent with a volume ratio of 0.01:1:4, and add to step (3 ) in the resulting reaction solution, kept stirring at 0°C for 60 minutes.
[0054] (5) After the reaction, the reaction solution obtained in step (4) was diluted with dichloromethane, washed with water three times, and the organic phase was coll...
Embodiment 3
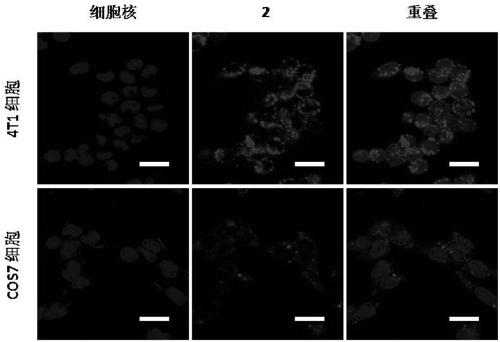
[0059] Breast cancer cells (4T1 cells) and African green monkey kidney cells (COS7 cells) were divided into 1 × 10 5 Cells / well were seeded into confocal small dishes and cultured in 1 mL RPMI-1640 medium at 37°C. After 24 hours, the anti-tumor compound 2 was dissolved in the medium, and 1 mL of the medium containing the anti-tumor compound 2 (20 μmol / L) was added to the breast cancer cells and Vero cells. After culturing for 2 hours, the medium containing the anti-tumor compound 2 was aspirated, the cells were washed three times with PBS buffer solution, and the nuclei were labeled with Hoechst 33342, and then the fluorescence intensity of the anti-tumor compound 2 in the cells was observed with a confocal laser microscope.
[0060] The result is as image 3 As shown, compared with the African green monkey kidney cells, the anti-tumor compound 2 has a better enrichment effect on breast cancer cells, indicating that the anti-tumor compound 2 has a good targeted enrichment eff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com