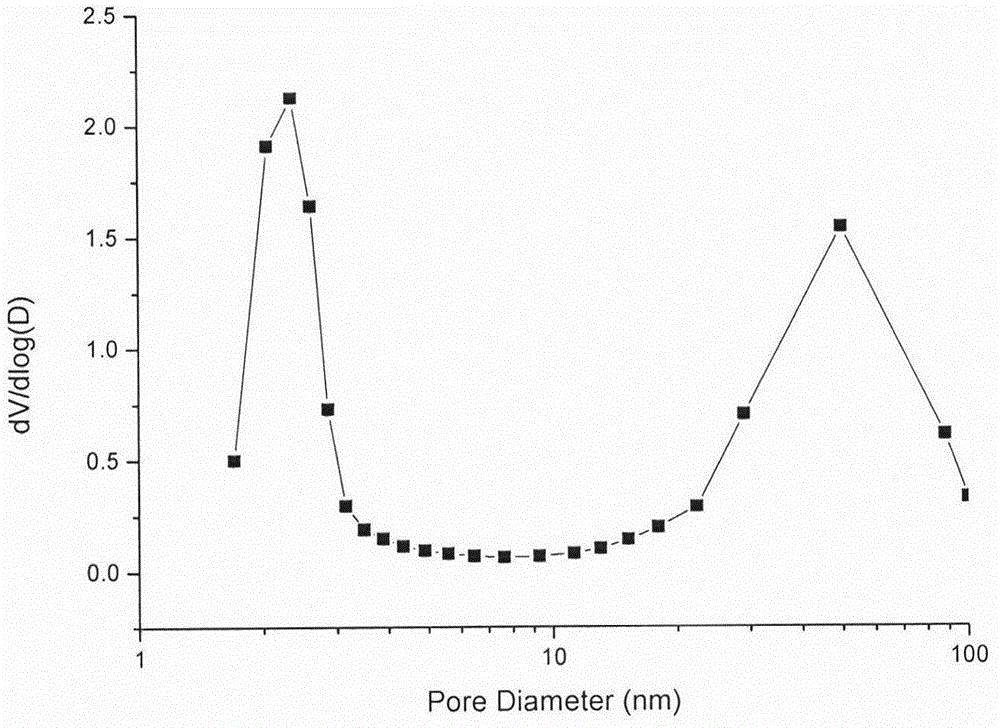
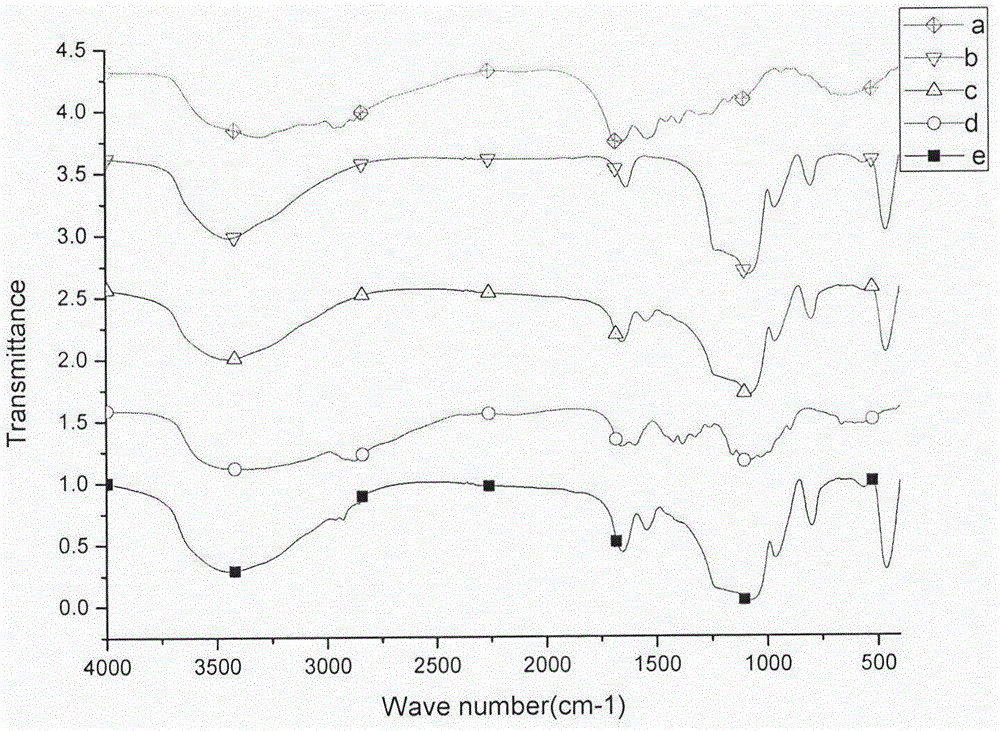
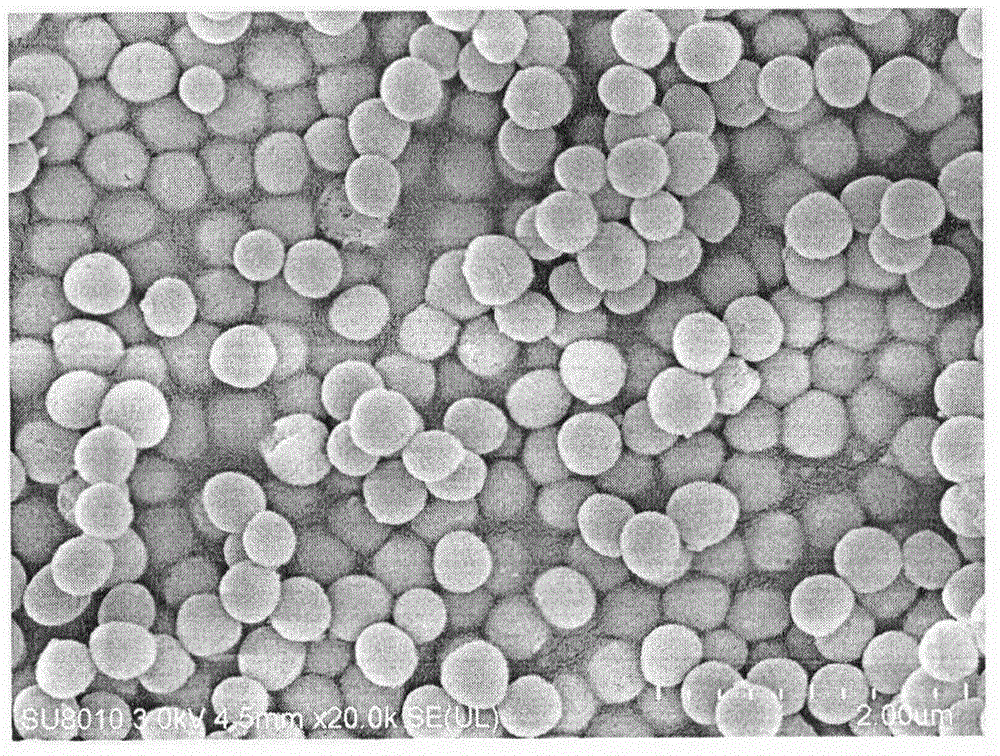
Preparation method for oral protein immune carrier
A protein vaccine and carrier technology, applied in the field of medicine, can solve the problems of inability to achieve prevention or treatment, protein vaccine inactivation, etc., and achieve the effect of protection stability, surface pores and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of mesoporous silica support:
[0034] (1) Accurately weigh 500mg of cetyltrimethylammonium bromide (CTAB), dissolve it in 70ml of deionized water, then add 0.8ml of ammonia solution, 20ml of absolute ethanol, and 20ml of ether, and stir vigorously at room temperature for 30 minutes After the solution became clear, 2.5 ml of tetraethylorthosilicate was quickly added dropwise thereto, and vigorously stirred for 4 hours to obtain a white solid precipitate.
[0035] (2) Suction filter the obtained white solid precipitate, wash the precipitate several times with ethanol and deionized water, and then dry it in a 60-degree oven for 24 hours.
[0036] (3) Accurately weigh 0.5 g of the dried solid powder, put it in 100 ml of ammonium nitrate / 95% ethanol with a concentration of 6 g / L, stir and reflux at 60 degrees for 5 hours, filter with suction, wash the precipitate, and dry in an oven , to obtain mesoporous silica.
Embodiment 2
[0038] Preparation of Mesoporous Silica Drug-loaded Particles:
[0039] (1) Accurately weigh 500mg of bovine serum albumin (BSA), put it in a 250mL volumetric flask, and dilute to the mark with phosphate buffer solution with a pH of 7.4 to obtain a BSA solution with a concentration of 2mg / mL as a stock solution.
[0040] (2) Accurately weigh 60 mg of mesoporous silica, add 30 ml of stock solution, stir at 4°C for 24 hours, centrifuge the solution, freeze-dry the precipitate, and obtain a freeze-dried powder of BSA loaded on mesoporous silica.
Embodiment 3
[0042] Preparation of chitosan mesoporous silica drug-loaded particles:
[0043] (1) Accurately measure 1 mL of acetic acid with a mass fraction of ≥99% in a 100 mL volumetric flask, and dilute to obtain a 1% acetic acid solution.
[0044] (2) Accurately weigh 50mg of chitosan, dissolve it in 10mL of 1% acetic acid solution, after it is completely dissolved, adjust its pH to about 5.5 with 2M sodium hydroxide solution, add deionized water to 25mL, and obtain the concentration 2mg / mL chitosan solution.
[0045](3) Accurately weigh 10 mg of mesoporous silica drug-loaded freeze-dried powder, add 1 mL of chitosan solution with a concentration of 2 mg / mL, stir at 4 degrees for 10 h, centrifuge the solution, and freeze-dry the precipitate to obtain chitosan Encapsulation of mesoporous silica drug-loaded particles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com