Preparation and application of reducing sensitive nano-micelle
A nanomicelle, sensitive technology, applied in the field of preparation and application of amphiphilic block polymers, can solve the problems of inability to release the drug and reduce the efficacy of the drug, and achieves improved bioavailability, improved stability, and is not easy to solve. away effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
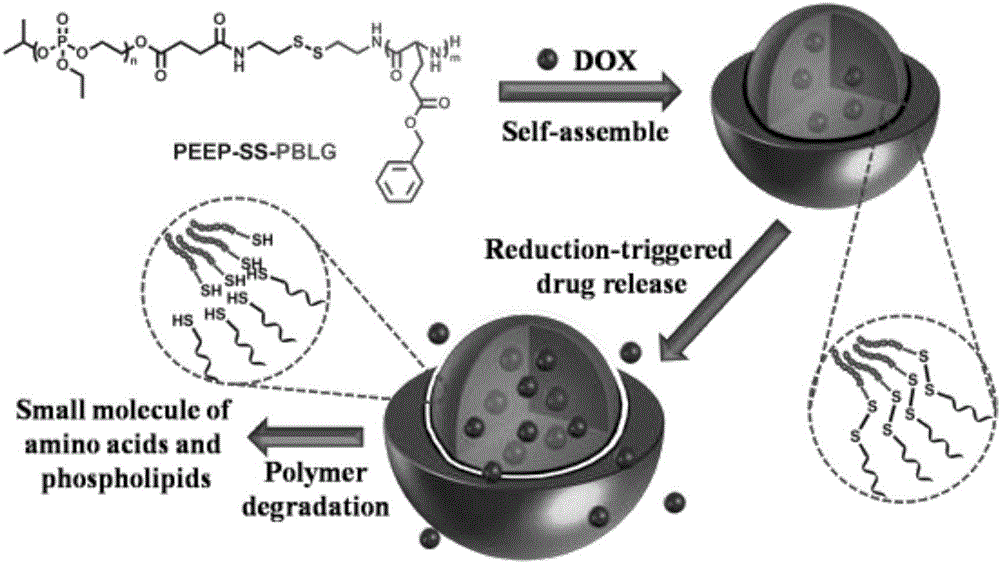
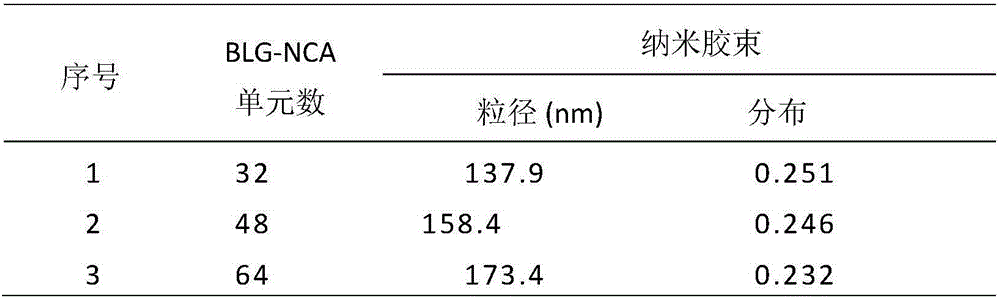
[0038] The preparation method of nano micelles: the amphiphilic block polymer is first dissolved in an organic solvent, and the secondary water with a mass percentage of 180%-270% is added dropwise to the polymer solution under stirring at room temperature; it is formed by self-assembly Nano micelles with polyphosphate as the hydrophilic shell and polyamino acid ester as the hydrophobic core; the particle diameter of the nano micelles is 10-300nm, and the particle diameter distribution PDI is 0.01-0.30.
[0039] The polymer solution is a solution of dimethyl sulfoxide, tetrahydrofuran or N,N-dimethylformamide with a polymer mass percentage concentration of 0.2%; the organic solvent includes: dimethyl sulfoxide, tetrahydrofuran or N, N-Dimethylformamide.
[0040] The amphiphilic block polymer is a reduction-responsive polyphosphate-polyamino acid block polymer, and has a method for preparing a reductive polyphosphate-polybenzyl glutamate amphiphilic block polymer Yes: At room ...
Embodiment 1
[0065] Embodiment 1: Synthetic cyclic phosphate monomer
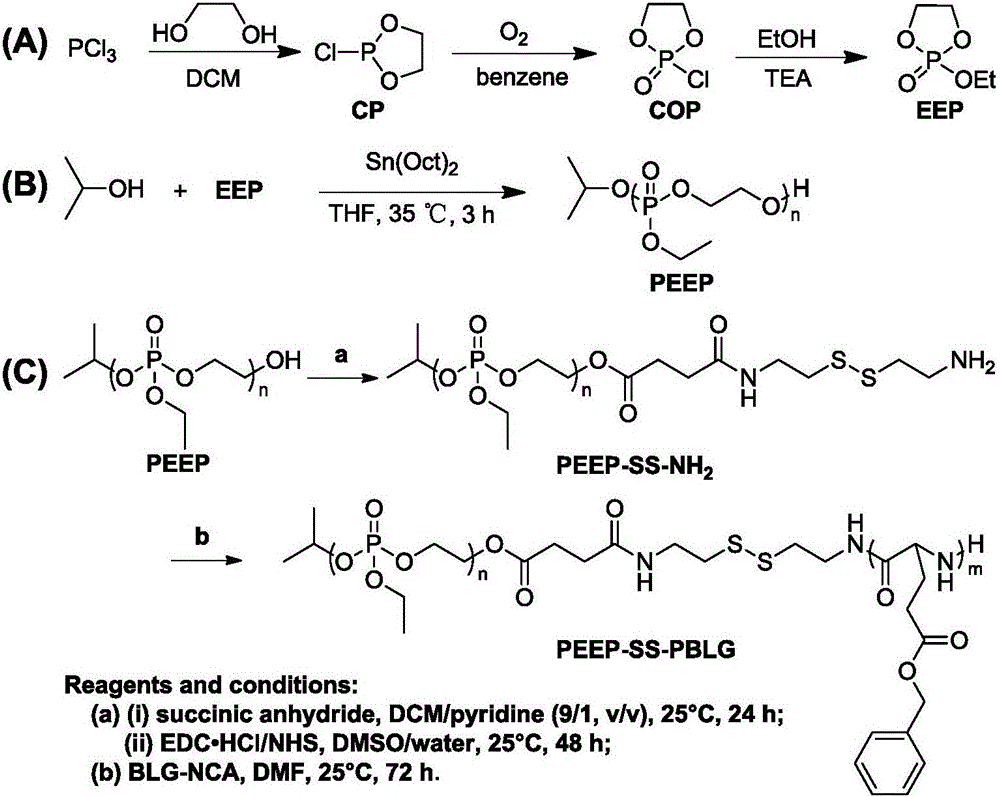
[0066] The cyclic phosphate monomer 2-ethoxy-2-oxo-1,3,2-dioxaphospholane (EEP) is based on phosphorus trichloride as a starting material, and ethylene glycol through nucleophilic Substitution and ring closure to form 2-chloro-1,3,2-dioxaphospholane (CP), and then react with oxygen to obtain 2-chloro-2-oxo-1,3,2-dioxaphospholane An important phosphorus oxychloride intermediate of pentane (COP). Finally, react COP with ethanol and use triethylamine (TEA) as an acid-binding agent to synthesize the cyclic phosphate monomer EEP. The specific operation is as follows:
[0067] Under nitrogen protection, 350 mL of anhydrous dichloromethane and phosphorus trichloride (412.5 g, 3 mol) were added into the flask. After stirring evenly, ethylene glycol (186.0g, 3mol) was slowly added dropwise to the system under vigorous stirring, and reacted at room temperature for 30 minutes. After the reaction was stopped, the solvent was remo...
Embodiment 2
[0070] Example 2: Synthesis of Polymer PEEP-SS-NH 2
[0071] The macromolecular initiator is based on small molecule isopropanol as an initiator, stannous octoate Sn(Oct) 2 As a catalyst, it is obtained by ring-opening polymerization of cyclic phosphate monomers to synthesize polyphosphate PEEP-OH at the hydroxyl end, and then sequentially reacting with succinic anhydride (SA) and cysteamine.
[0072] Under nitrogen protection, isopropanol (191.4mg, 3.19mmol) and EEP monomer (10g, 65.8mmol) were dissolved in 48mL of anhydrous THF, and stannous octoate (324.2mg, 0.8mmol) was added to the system at 35°C After stirring and reacting for 3 h, the system was settled in methanol / glacial ether (v / v, 1:10), and vacuum-dried at room temperature to obtain the polymer PEEP. The yield was 73.5%.
[0073] Under the protection of nitrogen, the hydroxyl-terminated polyphosphate PEEP-OH (5.5g, 1.0mmol) and succinic anhydride (0.12g, 1.2mmol) were dissolved in a mixed solution of 50mL dichlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com