Enzyme linked immunosorbent assay instrument testing method for penicillium marneffei mycelia-phase in-vitro susceptibility testing
A Marneffei and Penicillium technology, applied in the field of Penicillium marneffei detection, can solve the problems of error in experimental results, difficulty in obtaining accurate results due to different personal judgments, and difficulty in judging breakpoints.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Prepare antibiotic liquid, accurately weigh 1.0mg of ketoconazole, dissolve it with 2mL dimethyl sulfoxide solvent to make antibiotic liquid;
[0021] Use RPMI 1640 culture medium to dilute the antibiotic solution to 4 μg / mL, then use a sterile microporous membrane (0.22 μm) to filter it for later use;
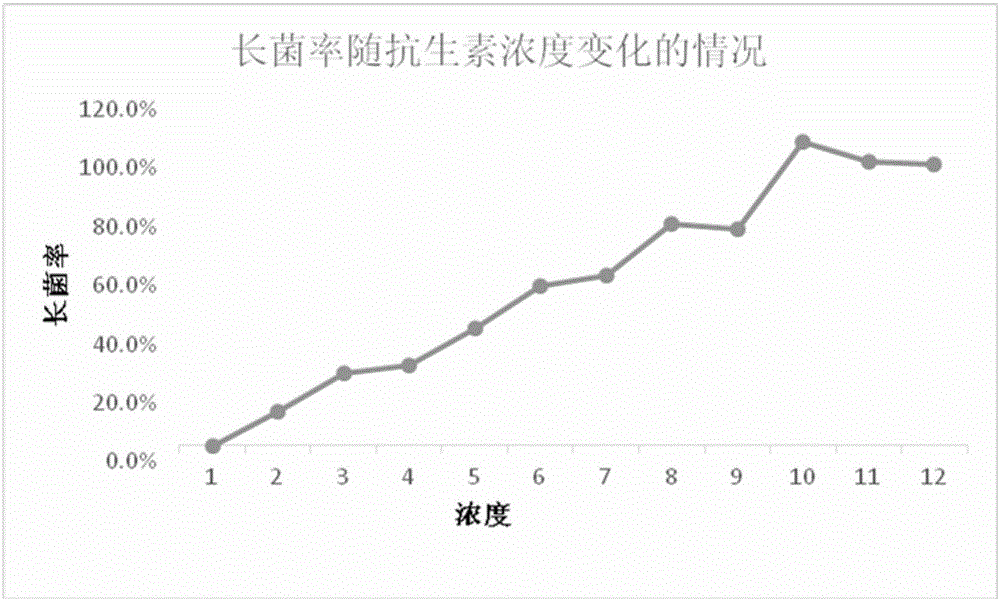
[0022]
[0023] Purification and cultivation of Penicillium marneffei: the lyophilized powder of Penicillium marneffei was cultivated and purified on PDA medium by dilution coating method;
[0024] Preparation of spore liquid: after the purified spores of Penicillium marneffei mature on the plate, draw 100-500 μL of RPMI 1640 culture solution with the tip of a sterile pipette and roll it on the plate to collect the spores. Collect into 10mL sterile plastic tube for use;
[0025] Cell counting of spore liquid: add the liquid in the plastic tube containing spores to 10mL, mix well, pipette 1-5μL liquid onto the hemocytometer plate, cover the glass slide and count und...
Embodiment 2
[0032] Prepare antibiotic liquid, accurately weigh 1.0mg of amphotericin B, and dissolve it with 2mL dimethyl sulfoxide solvent to make antibiotic liquid;
[0033] Use RPMI 1640 culture medium to dilute the antibiotic solution to 4 μg / mL, then use a sterile microporous membrane (0.22 μm) to filter it for later use;
[0034]
[0035] Purification and cultivation of Penicillium marneffei: the lyophilized powder of Penicillium marneffei was cultivated and purified on PDA medium by dilution coating method;
[0036] Preparation of spore liquid: after the purified spores of Penicillium marneffei mature on the plate, draw 100-500 μL of RPMI 1640 culture solution with the tip of a sterile pipette and roll it on the plate to collect the spores. Collect into 10mL sterile plastic tube for use;
[0037] Cell counting of spore liquid: add the liquid in the plastic tube containing spores to 10mL, mix well, pipette 1-5μL liquid onto the hemocytometer plate, cover the glass slide and cou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com