Method for synthesizing beta-lactamase inhibitor Avibactam
A technology of beta-lactamase and a synthesis method, which is applied in the field of preparation of beta-lactamase inhibitors, can solve the problems of cumbersome route process, low final yield, difficult operation, etc. Handling simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
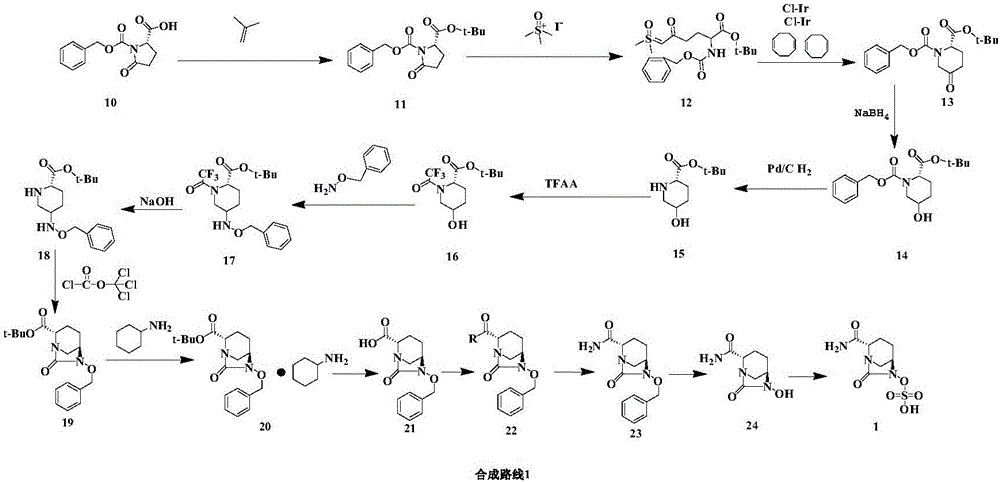
Embodiment 1
[0032] (1) Compounds Synthesis:
[0033] Add (S)-5-oxo-2 piperidine carboxylic acid (25.0g, 174.6mmol), dichloromethane (300ml) to the there-necked flask and stir, add 2,6-lutidine (1g, 9mmol), Benzylamine hydrochloride (26.5g, 184.8mmol) was heated to reflux for 2h, washed with purified water and saturated sodium chloride solution, separated and concentrated to give compound 3 (42.83g, 171.1mmol, yield 98%) as light brown oil )
[0034] (2) Compounds Synthesis:
[0035] Add purified water (600ml), glucose (20g), citric acid (0.5g), PEG 2000 (0.1g), disodium hydrogen phosphate (0.5g), dry baker's yeast (40g) to a three-necked bottle, stir at a constant temperature of 30°C For 1 h, add compound 3 (40 g, 172.2 mmol) in ethyl acetate solution (300 ml) in three batches with 1 h interval between each batch. After the addition, the reaction was incubated at 30 °C for 24 h, filtered, separated, and the aqueous phase was extracted with ethyl acetate. , the organic phases were c...
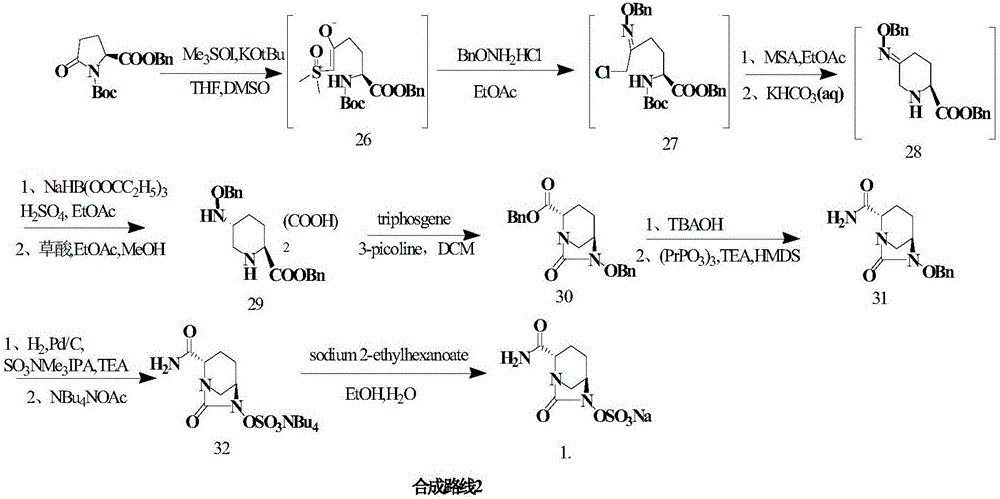
Embodiment 2
[0049] Others are the same as in Example 1, except:
[0050] (1) In the synthesis of compounds , compound 2 and R 1 NH 2 The reaction produces compound 3, R 1 It is Teoc; it is heated to reflux for 1h (yield 96%).
[0051] (2) In the synthesis of compounds At the time, glucose was replaced with sucrose; the organic solvent ethyl acetate was replaced with acetone; after adding the raw materials, the reaction was incubated at 5°C for 72 h (yield 94%, optical activity ee value 99%).
[0052] (3) In the synthesis of compounds When , trifluoroacetic anhydride was added dropwise to react for 0.5 h, and the temperature was maintained at -20°C (yield 96%).
[0053] (4) In the synthesis of compounds , after adding benzyloxyamine, maintain the temperature for 1h, wherein R 1 for Teoc, R 2 is Alloc (96% yield).
[0054] (5) In the synthesis of compounds when (where R 1 for Teoc, R 2 For Alloc), the potassium carbonate solution was replaced with sodium hydroxide solution...
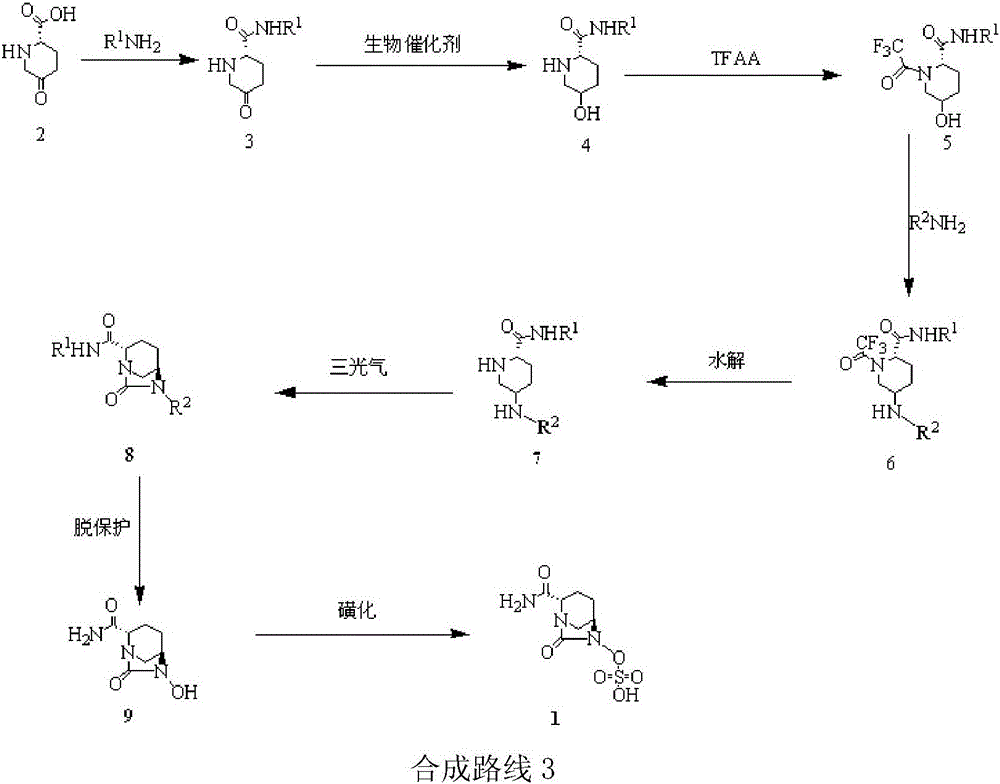
Embodiment 3
[0059] Others are the same as in Example 1, except:
[0060] (1) In the synthesis of compounds , compound 2 and R 1 NH 2 The reaction produces compound 3, R 1 is Dmb; the temperature is refluxed for 20h (yield 97%).
[0061] (2) In the synthesis of compounds When , the glucose was replaced with sucrose; the organic solvent ethyl acetate was replaced with dichloromethane; after adding the raw materials, the reaction was incubated at 40° C. for 1 h (yield 95%, optical activity ee value 98%).
[0062] (3) In the synthesis of compounds When , trifluoroacetic anhydride was added dropwise to react for 12 h, and the temperature was maintained at 0 °C (yield 96%).
[0063] (4) In the synthesis of compounds , after adding benzyloxyamine, maintain the temperature for 10h, wherein R 1 for Teoc, R 2 is Fmoc (96% yield).
[0064] (5) In the synthesis of compounds when (where R 1 for Teoc, R 2 For Fmoc), the potassium carbonate solution was replaced with triethylamine solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com