Optimized method for producing methacrolein
A technology for methacrolein and formaldehyde, applied in chemical instruments and methods, carboxylate preparation, carboxylate preparation, etc., can solve the problems of expensive, inconvenient, colored secondary products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0045] The method according to the invention and the advantages resulting therefrom are further described below with reference to these examples, which however should not be construed as limiting the invention in any way.
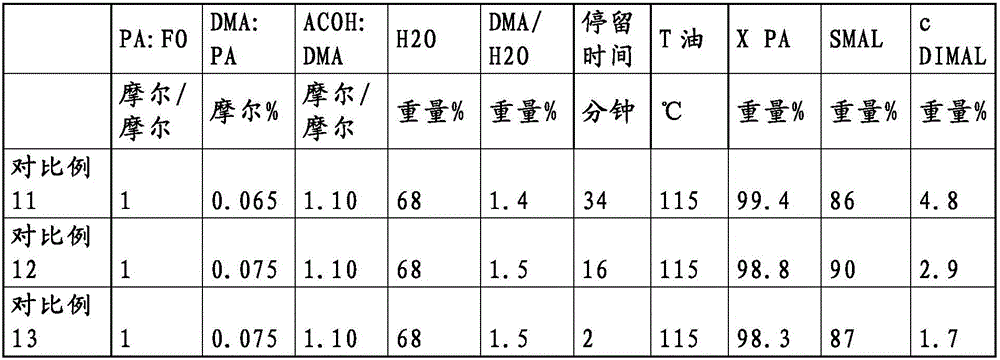
[0046] Examples 1 to 9 / Comparative Examples 1 to 11 (see Table 1).
[0047] Depending on the example, a formalin solution with a formaldehyde content of 37% by weight or 55% by weight was mixed with propionaldehyde by means of a static mixer (hereinafter referred to as aldehyde solution) and mixed in an oil-heated heat exchanger. The mixture was then heated to the desired temperature (see Table 1). Depending on the example, the exact water content of the formalin plays no further role, since according to Table 1 it is fully incorporated into the water content of the fresh feed. The recycle stream from the bottom of the product column connected after the tubular reactor was mixed with acetic acid and dimethylamine (as a 40% solution in water) and likewise p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com