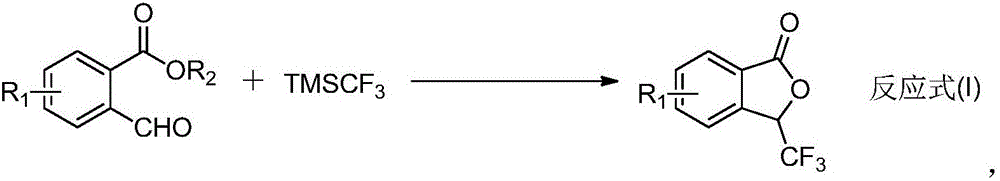
Preparation method of 3-trifluoromethyl phthalide
A technology of trifluoromethylphthalide and trifluoromethyltrimethylsilane is applied in the field of preparation of 3-trifluoromethylphthalide, can solve the problems of complicated operation, low reaction yield and the like, and achieves high reaction activity , the reaction operation is simple, the effect of low environmental damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] At room temperature, methyl 2-formylbenzoate (1mmol), TMSCF 3 (1.2mmol) and potassium carbonate (1.0mmol) were miscible in 2mL DMF, and the mixture was stirred and reacted for 8h. After the reaction, water and ethyl acetate were added, and the organic phase was obtained by extraction, dried, and the organic solvent was removed by distillation under reduced pressure. The product 3-trifluoromethylphthalide was obtained by column chromatography with a yield of 87%.
Embodiment 2
[0016] Reaction steps are identical with embodiment 1, difference is:
[0017] The amount of potassium carbonate used was 0.75 mmol, and the yield of 3-trifluoromethylphthalide was 84%.
Embodiment 3
[0019] Reaction steps are identical with embodiment 1, difference is:
[0020] The amount of potassium carbonate used was 0.5 mmol, and the yield of 3-trifluoromethylphthalide was 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com