Preparation method for platinum-copper nano-particles with controllable morphologies
A nanoparticle, platinum-copper technology, applied in the field of nanomaterials, can solve the problems of high price and inactivation of platinum in fuel cells, and achieve the effects of easy operation and implementation, uniform size and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A method for preparing platinum-copper nanoparticles with controllable morphology, comprising the steps of:
[0046] (1) Weighing medicines
[0047] Weigh 0.0200g of platinum acetylacetonate and 0.0100g of copper acetylacetonate into a 50ml three-necked round-bottomed flask, and then pipette 2.0mL of oleylamine, 1.0mL of oleic acid, and 7mL of dibenzyl ether. The magnet is placed at the bottom of the bottle. It should be noted that the reaction was performed in a fume hood, which will not be described below.
[0048] (2) Nitrogen gas
[0049] Nitrogen was passed into the closed device system of the three-necked flask to check the airtightness of the device.
[0050] (3) Reaction condition setting
[0051] Turn on the heating switch of the heating mantle and set the heating temperature to 130°C. This temperature was maintained for 30 minutes.
[0052] (4) Pass CO gas
[0053] Stop feeding nitrogen, start feeding CO gas, the gas purity is 99.999%, the gas flow rate i...
Embodiment 2
[0062] A method for preparing platinum-copper nanoparticles with controllable morphology, comprising the steps of:
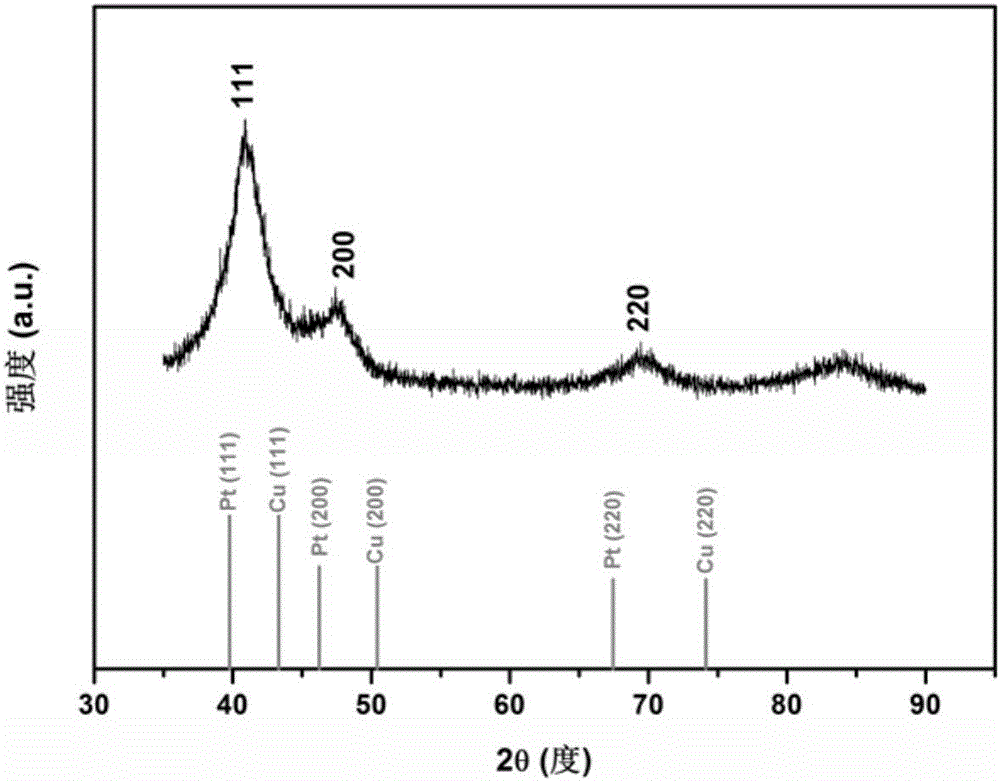
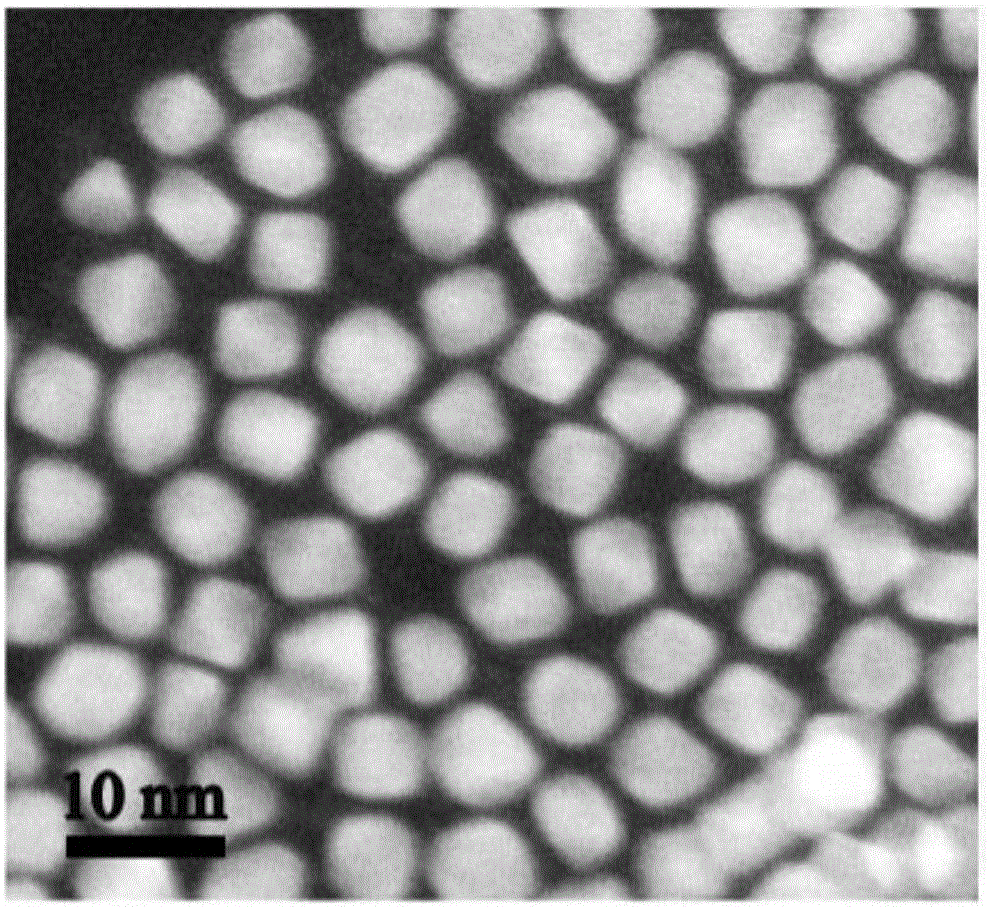
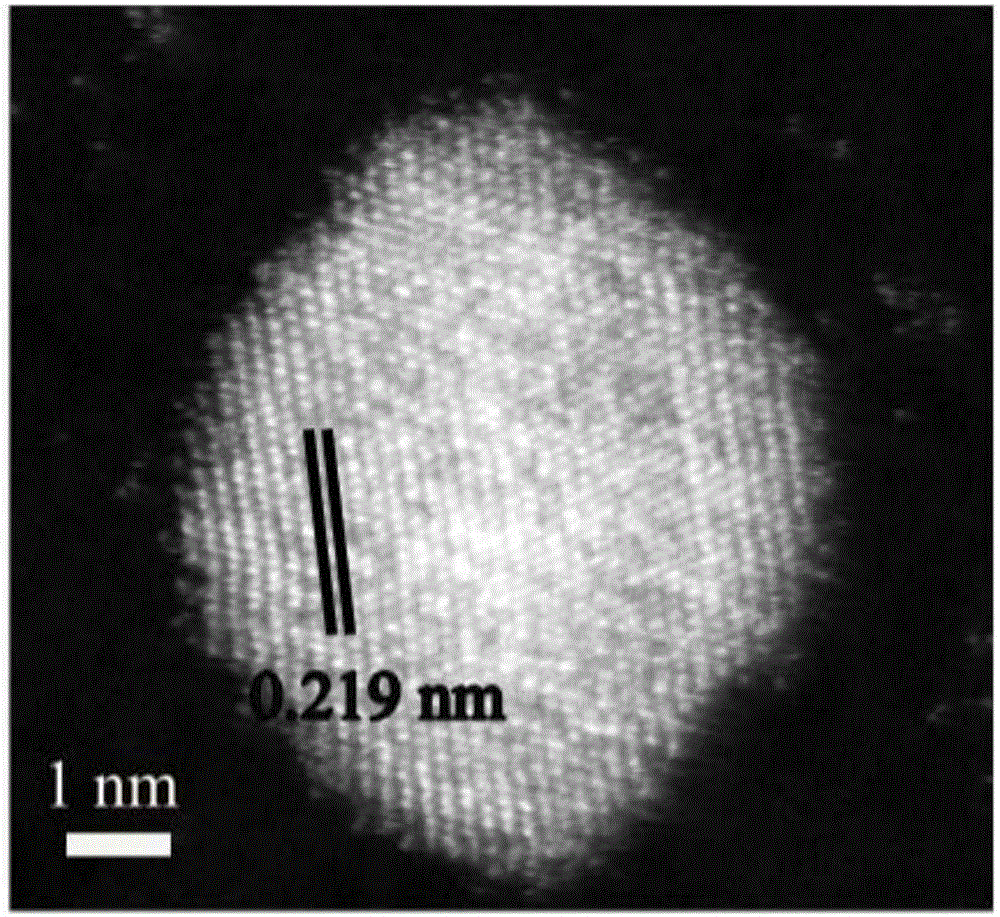
[0063] Weigh 0.0100g of platinum acetylacetonate and 0.0100g of copper acetylacetonate into a 50ml three-necked round-bottomed flask, then pipette 2.0mL of oleylamine, 1.0mL of oleic acid, and 7mL of dibenzyl ether. Under the condition of nitrogen protection, the mixed system was heated and stirred at 130°C for 30min, the nitrogen gas was stopped, and the CO gas was started to flow in at a gas rate of 100mL / min, the reaction temperature was set at 230°C, and the reaction time was 40min. The whole reaction process was kept under the protection of CO. Cool to room temperature, wash with n-hexane and ethanol, and centrifuge until the supernatant liquid is colorless and clear. Take the bottom precipitate and disperse it in 10 ml of n-hexane for storage to obtain a stable dispersion of platinum-copper nanoparticles. The morphology of the obtained platinum-copper na...
Embodiment 3
[0065] A method for preparing platinum-copper nanoparticles with controllable morphology, comprising the steps of:
[0066] Weigh 0.0500g of platinum acetylacetonate and 0.0100g of copper acetylacetonate into a 50ml three-neck round-bottomed flask, then pipette 2.0mL of oleylamine, 1.0mL of oleic acid, and 7mL of dibenzyl ether. Under the condition of nitrogen protection, the mixed system was heated and stirred at 130°C for 30min, the nitrogen gas was stopped, and the CO gas was started, the gas flow rate was 100mL / min, the reaction temperature was set at 230°C, and the reaction time was 40min. The whole reaction process was kept under the protection of CO. Cool to room temperature, wash with n-hexane and ethanol, and centrifuge until the supernatant liquid is colorless and clear. Take the bottom precipitate and disperse it in 10 ml of n-hexane for storage to obtain a stable dispersion of platinum-copper nanoparticles. The morphology of the obtained platinum-copper nanoparti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Lattice constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com