Biomarker for detecting occlusion or stenosis of coronary artery and preparation method thereof, and reagent kit containing biomarker
A biomarker, coronary artery technology, applied in biochemical equipment and methods, microbial determination/examination, DNA/RNA fragments, etc., can solve problems such as affecting accuracy, and achieve ideal accuracy, high sensitivity and specificity. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1c
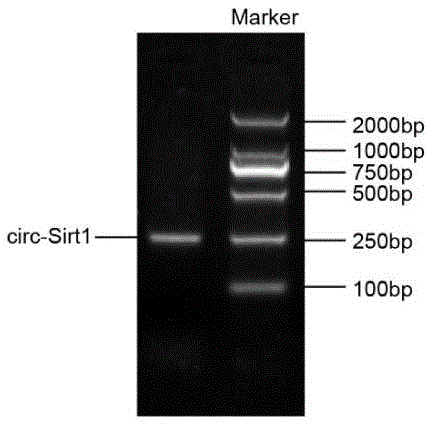
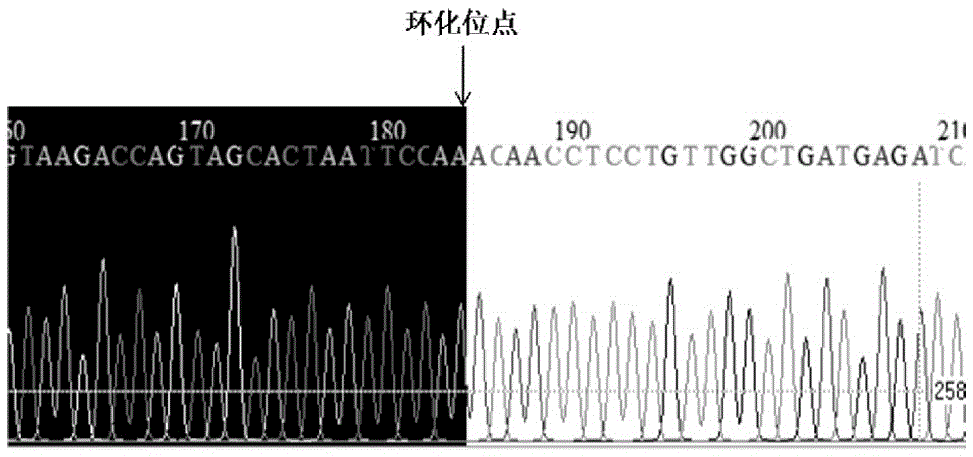
[0056] Preparation of embodiment 1circ-Sirt1 gene
[0057] 1) Extraction of total RNA in human plasma: extraction using Trizol LS Reagent reagent based on Life Technologies.
[0058] (a) Using EDTA-K 2 Anticoagulant tubes were used to collect peripheral venous blood samples. Within 2 hours of the collected blood sample, centrifuge at 2000g at 4°C for 20min, collect the supernatant and centrifuge again at 10000g at 4°C for 20min, and store the supernatant at -80°C.
[0059] (b) Add 900 μl Trizol LS to 300 μl plasma, mix well and lyse at room temperature for 5 minutes.
[0060] (c) Add 0.2ml of chloroform:isoamyl alcohol (24:2), shake for 15s, and let stand at room temperature for 2min.
[0061] (d) Centrifuge at 12000 g for 15 min at 4°C, and take the supernatant.
[0062] (e) Add 0.6ml of isopropanol, mix the liquid in the tube gently, and let stand at -20°C for 2h.
[0063] (f) Centrifuge at 12000 g for 15 min at 4°C, and take the supernatant.
[0064] (g) Add 1 ml of 7...
Embodiment 2
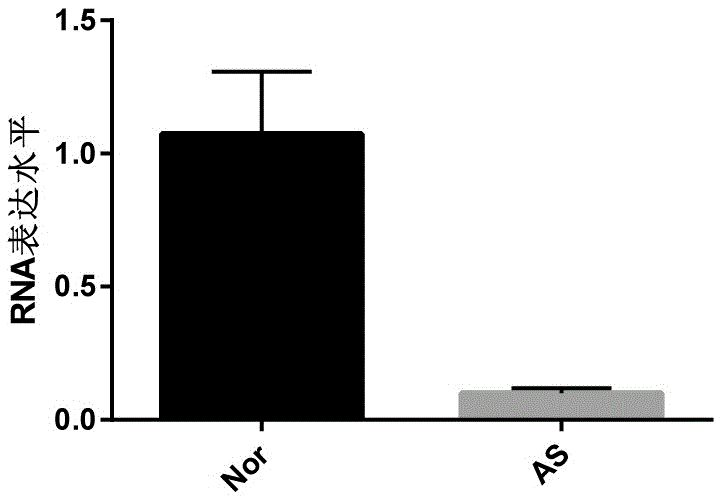
[0076] Example 2 Using the biomarkers of the present invention to detect and evaluate the degree of stenosis of the coronary arteries of patients
[0077] Blood samples: Plasma samples from 20 patients with coronary atherosclerosis came from inpatients in the Department of Cardiology, The Second Affiliated Hospital of Hebei Medical University. Plasma samples from 20 healthy individuals were obtained from the Physical Examination Center of the Second Affiliated Hospital of Hebei Medical University.
[0078] 1) Inclusion criteria
[0079] Patients with initial or reoccurrence of coronary artery sclerosis occlusive stenosis obtained by coronary angiography.
[0080] 2) Exclusion criteria
[0081] Healthy people: healthy people of similar age, no history of coronary heart disease, no history of surgery, normal blood lipids, blood sugar, blood pressure, no family genetic diseases, no liver and kidney insufficiency;
[0082] 3) Grouping of collected specimens
[0083] (a) Experi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com