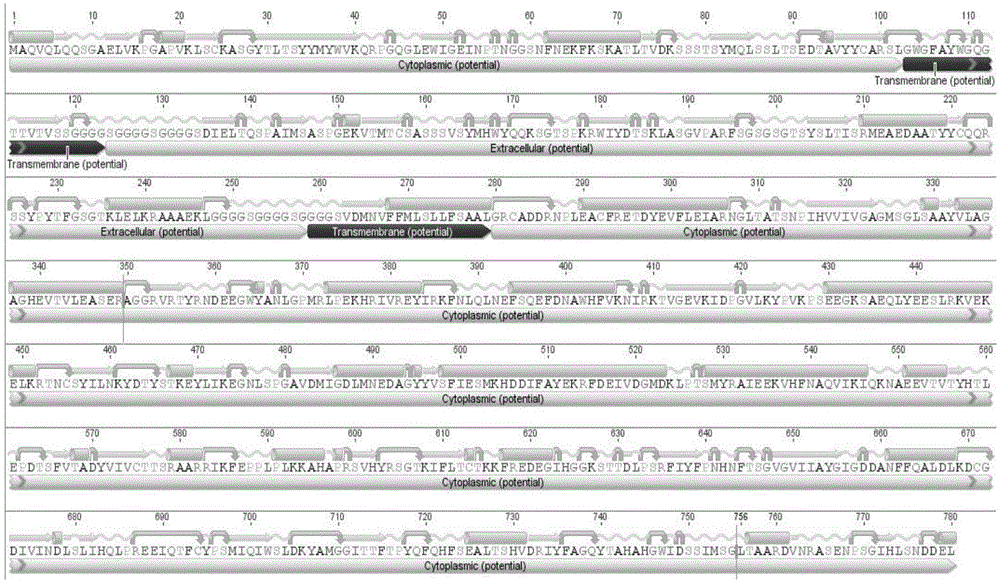
Anti-IL-4R single-chain antibody and snake venom L-amino acid oxidase fusion protein and application thereof
A single-chain antibody, fusion protein technology, applied in the biological field, can solve problems such as short half-life and clinical application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
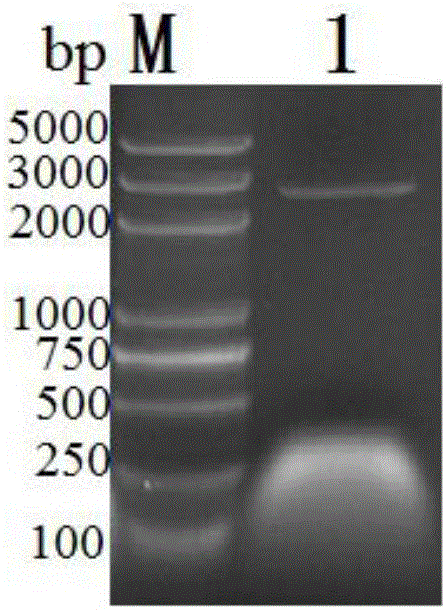
[0112] A fusion protein of anti-IL-4R single-chain antibody and snake venom L-amino acid oxidase, the preparation of the fusion protein specifically includes the following steps:
[0113] (1) Select the scFv gene sequence whose restriction sites are EcoRI and XhoI, and connect the scFv and LAAO genes by overlapping extension PCR technology. Add an XhoI restriction site at the 3' end of the venom LAAO gene sequence, add an overlapping sequence identical to the scFv gene sequence of the anti-IL-4R single-chain antibody, and a linking peptide linking gene at the 5' end of the venom LAAO gene sequence. Add an XhoI restriction site at the 3' end of the "overlapping sequence" gene sequence;
[0114] Wherein, primer F1 and primer F2 are designed to remove the Xho I restriction site of the anti-IL-4R single-chain antibody scFv gene sequence;
[0115] (2) Design primers F1 and F3, remove 15 μL of scFv gene fragment of XhoⅠ restriction site, 15 μL of LAAO gene fragment, 1 μL of primer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com