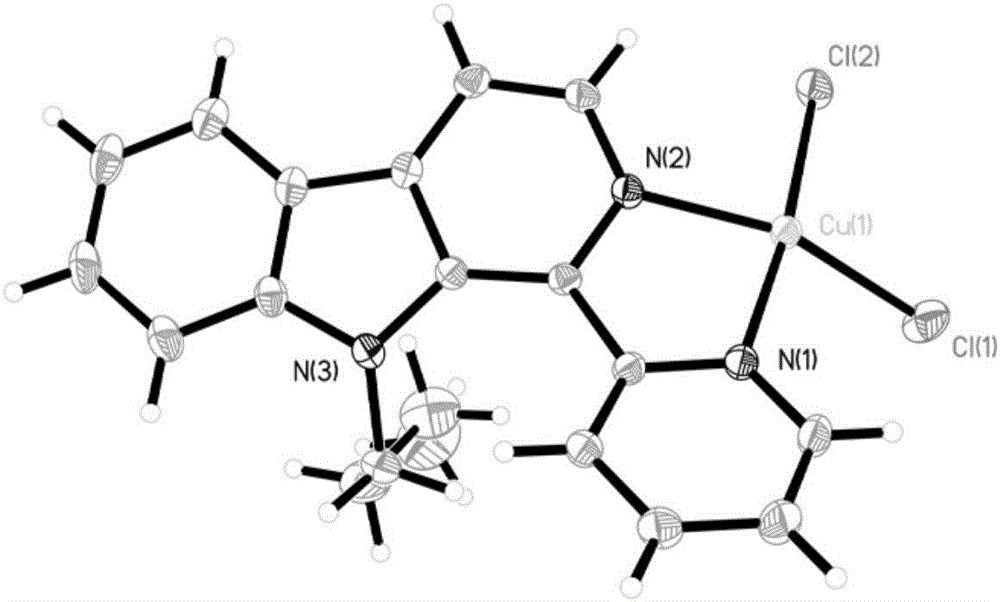
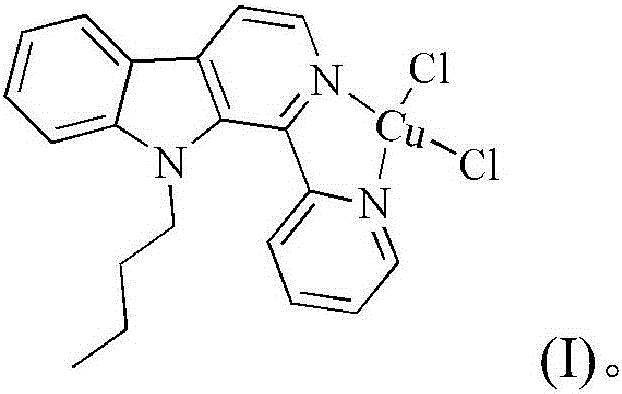
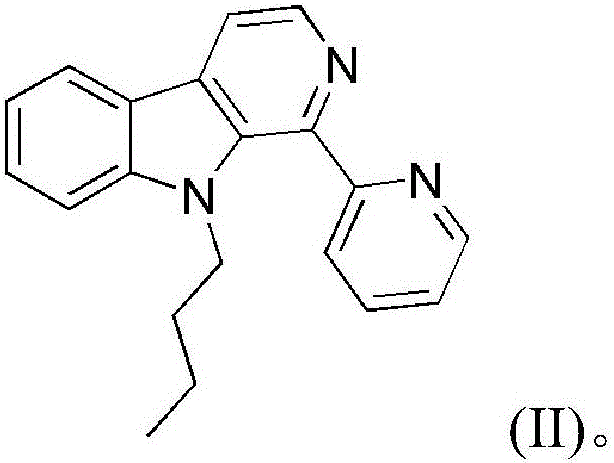
Copper chloride complex by taking 1-(2-pyridine)-9-butyl-beta-carboline as ligand as well as synthetic method and application of complex
A synthesis method and compound technology, applied in the field of medicine, can solve problems such as the synthesis method and application of copper chloride that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the compound shown in formula (II) is the synthesis of 1-(2-pyridine)-9-butyl-β-carboline (L)
[0045] 1) Add 1.6g (10mmol) tryptamine, 1.1g (10mmol) pyridine-2-carbaldehyde and 50ml toluene into a 150ml round bottom flask, add a water separator and a condenser tube to form a component water reflux device, heat and reflux for 4 hours, wait After the reaction, the solvent was evaporated to dryness, and the residue was recrystallized with 100ml of n-hexane to obtain compound 1 (2.3g, yield 92%);
[0046] 2) Add 2.5g (10mmol) compound 1, 2.5g palladium carbon (10%Pd / C) and 100ml p-xylene to a 250ml round bottom flask, heat to reflux, and use thin layer chromatography to track and detect until the end of the reaction, and let stand Suction filtration and the filtrate was evaporated to dryness, and the resulting residue was purified by silica gel column chromatography (V 石油醚 :V 二氯甲烷 =1:1), to obtain compound 2 (2.1g, yield 86%);
[0047] 3) Add 0.24g (10mmol...
Embodiment 2
[0055] Embodiment 2: the synthesis of ligand L
[0056] Repeat Example 1, the difference is:
[0057] In step 2), with Mn(Ac) 3 Instead of palladium carbon, glacial acetic acid instead of p-xylene, control Mn(Ac) 3 The amount of compound 1 added was twice that of compound 1, and the reaction was carried out at 70°C. TLC was tracked and detected until the end of the reaction, and then the pH of the system was adjusted to 7 with ammonia water, extracted three times with ethyl acetate, and the organic phases were combined. , evaporate the solvent to dryness, and the resulting residue is applied to a silica gel column (chromatographic purification (V 石油醚 :V 二氯甲烷 =1:1), compound 2 was obtained.
[0058] The product obtained in this example was analyzed by proton nuclear magnetic resonance, carbon nuclear magnetic resonance and high-resolution mass spectrometry, and it was determined to be the target product 1-(2-pyridine)-9-butyl-β-carboline.
Embodiment 3
[0059] Embodiment 3: the synthesis of ligand L
[0060] Repeat Example 2, the difference is:
[0061] In step 2), with Pb(Ac) 4 instead of Mn(Ac) 3 , to control Pb(Ac) 4 The amount of added is 5 times the amount of compound 1, and the reaction is carried out at 60°C.
[0062] The product obtained in this example was analyzed by proton nuclear magnetic resonance, carbon nuclear magnetic resonance and high-resolution mass spectrometry, and it was determined to be the target product 1-(2-pyridine)-9-butyl-β-carboline.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com