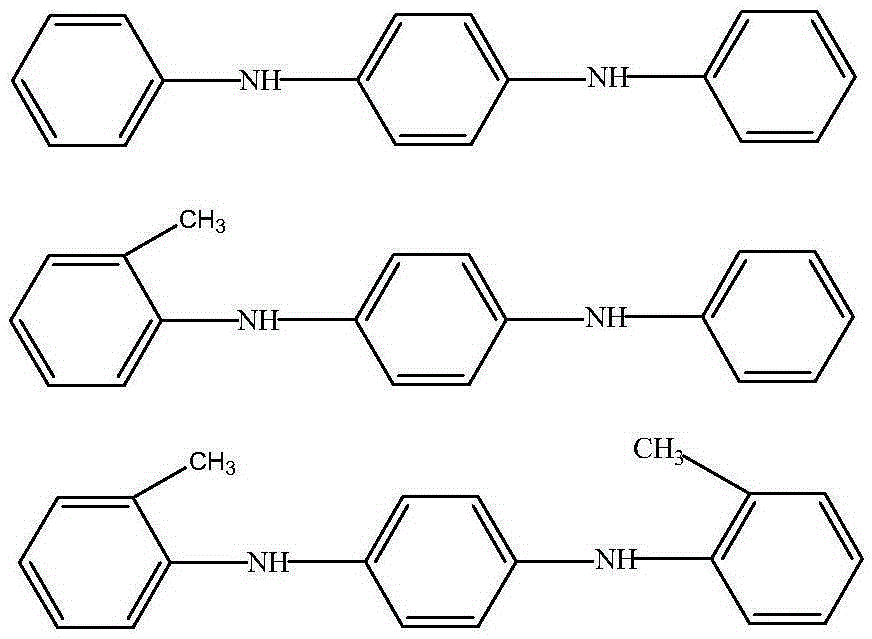
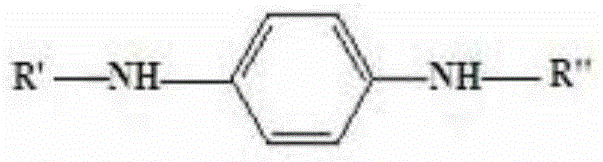
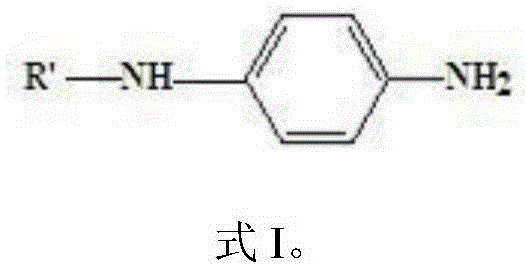
Preparation method for aryl substituted p-phenylenediamine substance
A technology of p-phenylenediamines and aryl groups, which is applied in the field of preparation of aryl-substituted p-phenylenediamines, and can solve the problems of high cost, strong corrosion, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method comprises: reacting raw material A and raw material B under the action of a hydrogen acceptor and a catalyst to form aryl-substituted p-phenylenediamine substances; raw material A has the structure shown in formula I, and raw material B is cyclohexanone and / or Or o-methylcyclohexanone, the hydrogen acceptor is a hydrogen acceptor that can be converted into raw material B by hydrogen;
[0029]
[0030] The above preparation method provided by the present invention reacts raw material A having the structure of formula I with raw material B (cyclohexanone and / or o-methylcyclohexanone) to generate aryl-substituted p-phenylenediamines. Specifically, both cyclohexanone and o-methylcyclohexanone can undergo a dehydrogenation reaction under the action of a catalyst, and at the same time replace a hydrogen atom on the amino group at the N' position in the raw material A, forming R" as phenyl or o-methyl The aryl group of phenyl is substituted p-phenylen...
Embodiment 1
[0043] Into a 500 mL autoclave were added 36.8 g (0.2 mol) of N-phenyl-p-phenylenediamine, 94.1 g (1 mol) of phenol, 2.0 g (0.02 mol) of cyclohexanone and 1.3 g of dry Pd / C (containing 5 wt% Pd) Catalyst; start stirring, replace with nitrogen for 3 times, then heat up to 220°C, keep warm for 6 hours, then cool down to room temperature and discharge, filter and recover the catalyst, distill the filtrate under reduced pressure to remove unreacted phenol and cyclohexanone, and obtain a distillation raffinate of 51.7 g, the content of N,N'-diphenyl-p-phenylenediamine was 94.1% as measured by sampling, and the calculated yield was 93.6%.
Embodiment 2 to 5
[0045] Using the same raw materials and process conditions as in Example 1 to prepare N,N'-diphenyl-p-phenylenediamine, the difference is that the relationship between the amount of each raw material is different (wherein the amount of N-phenyl-p-phenylenediamine is kept weight unchanged). Concrete dosage relation and product situation are as follows:
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com