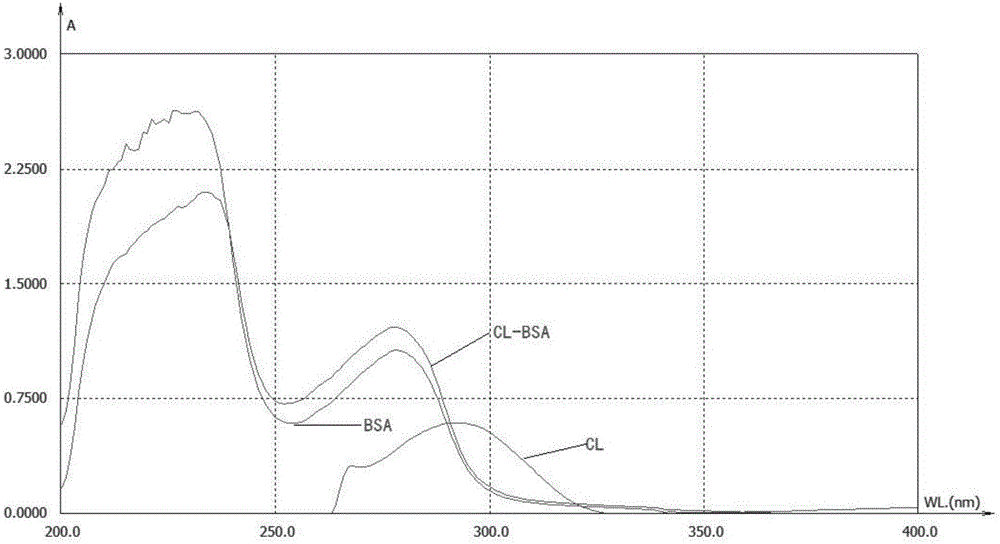
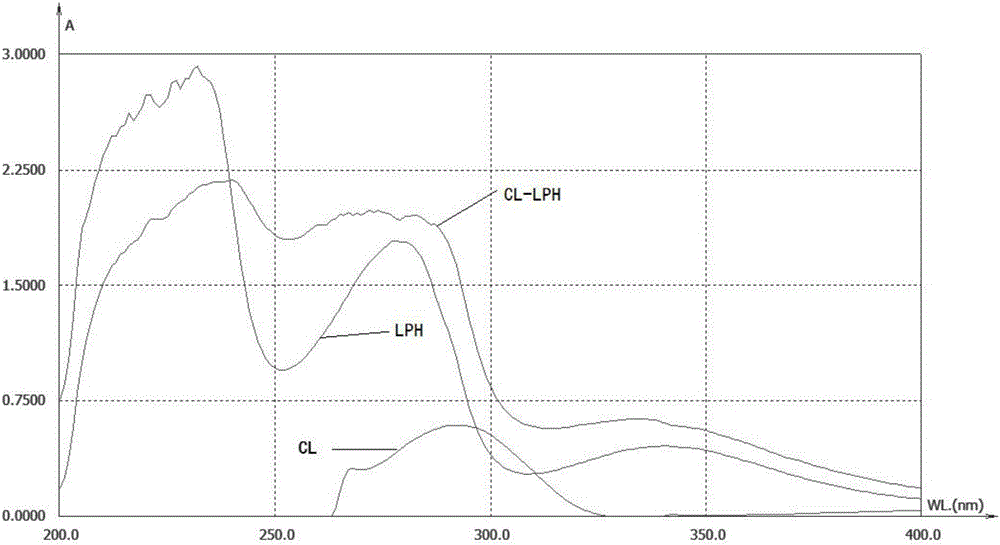
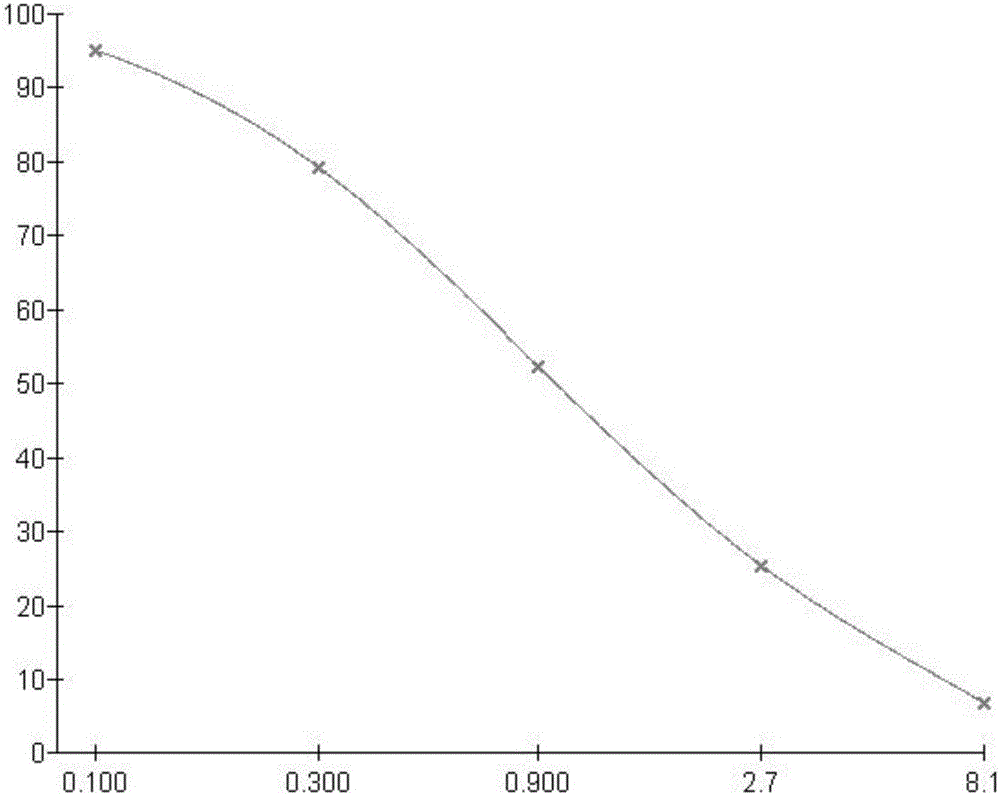
Clarithromycin hapten, artificial antigen and antibody, and preparation method and application thereof
A clarithromycin and hapten technology, which is applied in chemical instruments and methods, preparation of sugar derivatives, animal/human proteins, etc., can solve the problems of high cost, inapplicability to screening and detection of large batches of samples, cumbersome operation steps, etc., and achieve Low cost, high sensitivity, and the effect of analyzing large sample volumes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A preparation method of the clarithromycin hapten provided by the present invention is as follows: add 748.3 mg (1 mmol) of clarithromycin into a 50 ml reaction bottle, and add 10 ml of anhydrous acetonitrile to dissolve it. Weigh 120.4mg (1.2mmol) of succinic anhydride into the reaction flask, and measure 1ml of triethylamine into the reaction flask. Room temperature, magnetic stirring, reaction for 4 hours. Afterwards, spin evaporate under reduced pressure, concentrate, add 50ml of water, adjust the pH to about 5 with glacial acetic acid, precipitate an off-white solid, filter, take the filter cake, and crystallize with 15ml of a mixed solvent (V ethyl acetate: V n-hexane = 1:2) , filtered, and dried to obtain 237.6 mg of product, the structural formula of which is formula (I). Example 2
Embodiment 2
[0043] A preparation method of the clarithromycin hapten provided by the present invention is as follows: add 748.3 mg (1 mmol) of clarithromycin into a 50 ml reaction bottle, and add 10 ml of anhydrous acetonitrile to dissolve it. Weigh 100.2 mg (1 mmol) of succinic anhydride into the reaction flask, and measure 1 ml of triethylamine into the reaction flask. Room temperature, magnetic stirring, reaction for 3 hours. Afterwards, spin evaporate under reduced pressure, concentrate, add 50ml of water, adjust the pH to about 5 with glacial acetic acid, precipitate an off-white solid, filter, take the filter cake, and crystallize with 15ml of a mixed solvent (V ethyl acetate: V n-hexane = 1:2) , filtered, and dried to obtain 216.2 mg of product, the structural formula of which was formula (I).
Embodiment 3
[0045] A preparation method of the clarithromycin hapten provided by the present invention is as follows: add 748.3 mg (1 mmol) of clarithromycin into a 50 ml reaction bottle, and add 10 ml of anhydrous acetonitrile to dissolve it. Weigh 150.7mg (1.5mmol) of succinic anhydride into the reaction flask, and measure 1ml of triethylamine into the reaction flask. Room temperature, magnetic stirring, reaction for 5 hours. Afterwards, spin evaporate under reduced pressure, concentrate, add 50ml of water, adjust the pH to about 5 with glacial acetic acid, precipitate an off-white solid, filter, take the filter cake, and crystallize with 15ml of a mixed solvent (V ethyl acetate: V n-hexane = 1:2) , filtered, and dried to obtain 241.5 mg of product, the structural formula of which is formula (I).
[0046]
[0047]
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com