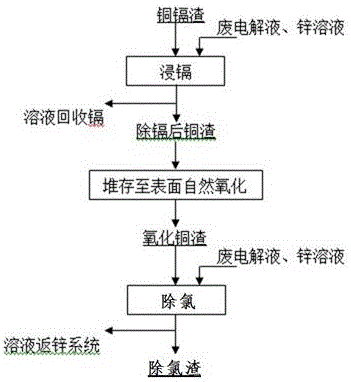
Method for removing chlorine from zinc solution by adopting cadmium removed and surface naturally-oxidized copper slag
A natural oxidation and copper oxidation technology, applied in the field of zinc hydrometallurgy, can solve the problems affecting the yield of zinc sheets, difficulty in stripping zinc sheets, and increased cathode power consumption, so as to improve the copper grade of copper slag and reduce the amount of copper slag added , The effect of simplifying the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Prepare a small amount of waste electrolyte in the zinc immersion solution to obtain a zinc sulfate solution with an initial sulfuric acid concentration of 10g / L, and add 10kg / m 3 Copper oxide slag on the surface without cadmium removal, heated to 60°C for 0.8h, the filtered solution contained chloride ions <200mg / L, the data is as follows:
[0024] g / L Cl Zn Cu Cd Pre-chlorination solution 1.06 145 0.30 0.24 After chlorine removal 0.17 147 1.65 0.53
Embodiment 2
[0026] A small amount of waste electrolyte is prepared in the zinc immersion solution to obtain a zinc sulfate solution with an initial sulfuric acid concentration of 10g / L, and 3kg / m 3 Copper oxide slag on the surface without cadmium removal, heated to 60°C for 0.8h, the filtered solution contains >300mg / L of chloride ions, the data is as follows:
[0027] g / L Cl Zn Cu Cd Pre-chlorination solution 1.06 145 0.30 0.24 After chlorine removal 0.55 146 2.14 0.47
Embodiment 3
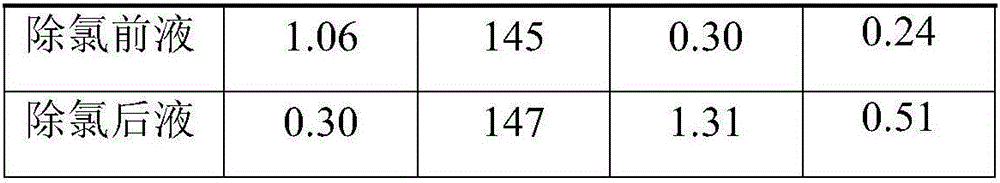
[0029] Prepare a small amount of waste electrolyte in the zinc immersion solution to obtain a zinc sulfate solution with an initial sulfuric acid concentration of 5g / L, and add 10kg / m 3 Copper oxide slag on the surface without cadmium removal, heated to 60°C for 0.8h, the filtered solution contained 300mg / L of chloride ions, the data is as follows:
[0030]
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com