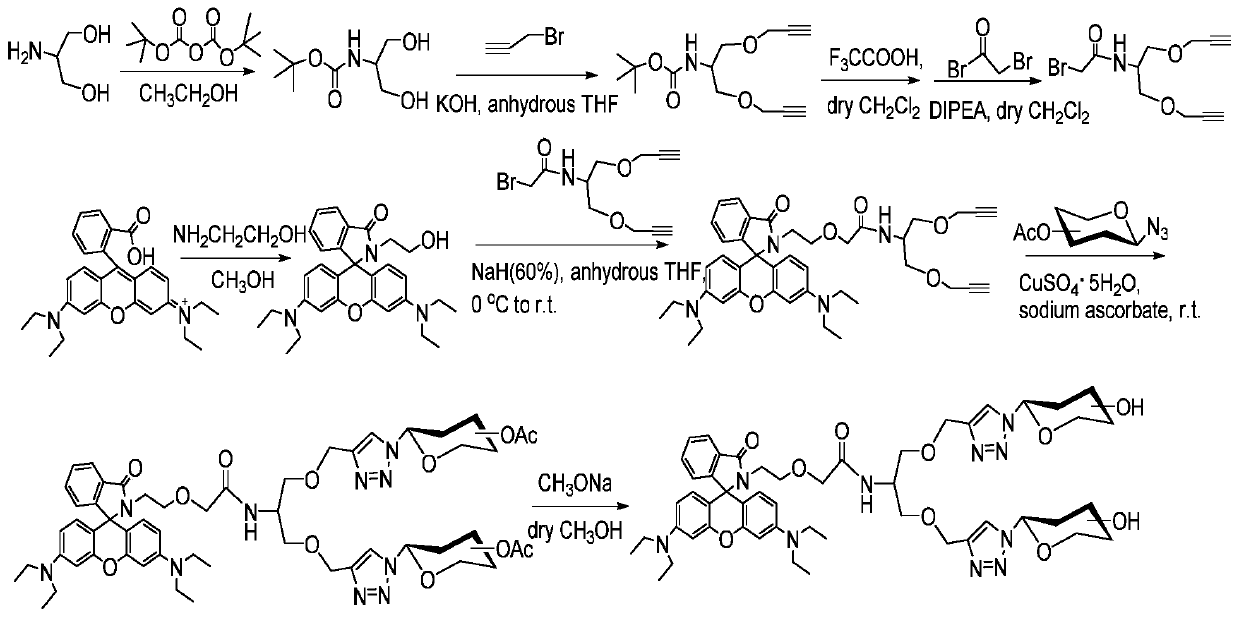
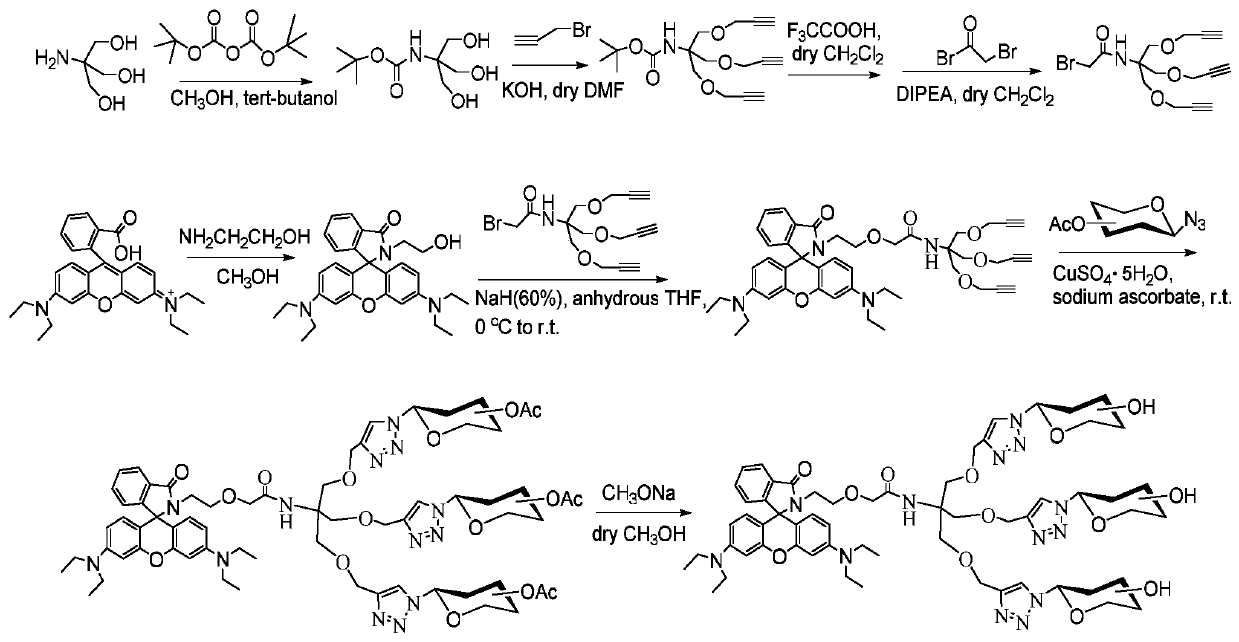
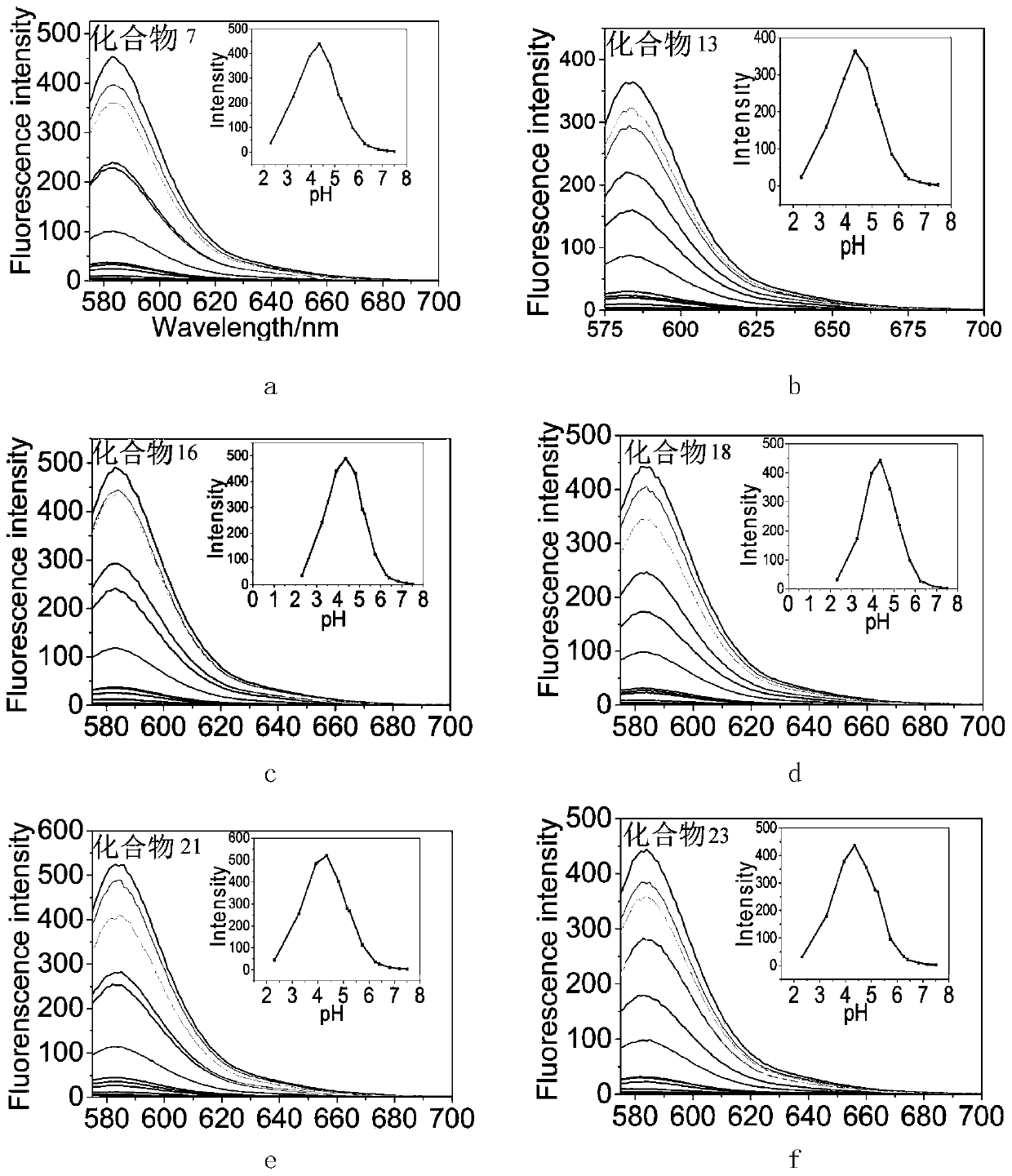
Two/three cluster glycosyl rhodamine derivatives and its preparation method and application
A derivative and reaction technology, applied in the field of two/three-cluster glycosyl rhodamine derivatives and their preparation, can solve the problems of poor water solubility and limitation of rhodamine, achieve good water solubility, strong hydrophilicity, and simple synthesis method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037] Add acetic anhydride (30mL) in a 100mL three-necked flask, add 3 drops of 70% perchloric acid dropwise under ice-water bath, stir for ten minutes, add D-galactose (5.00g) in batches, control the reaction temperature at 30- 40°C, after reacting for 1.5h, add red phosphorus (3.75g) when the reaction solution was cooled to 15°C, and stir for 10 minutes, then slowly add liquid bromine (2.8 mL) dropwise, and control the temperature below 20°C, dropwise After completion, stir at 15-20°C for 0.5h, continue to control the temperature below 20°C and slowly add deionized water (4.5mL), react at 15-20°C for about 0.5h, leave it at room temperature for 2h, add CH 2 Cl 2 (40mL), filter with suction, pour the filtrate into 200mL ice water, separate the organic layer, and wash the water layer with CH 2 Cl 2 Extract (4×30 mL), combine the organic layers, and wash the organic phase with saturated NaHCO 3 The solution was washed to neutrality, then washed with saturated br...
Embodiment 2
[0053]
[0054] Dissolve 5g of trishydroxymethylaminomethane in a mixed solution composed of 30mL of methanol and 30mL of tert-butanol, add BOC anhydride (11.75g) tert-butanol (50mL) solution under stirring, react overnight at room temperature, spin off the solvent, add ethyl acetate The ester was refrigerated overnight and filtered to give 8 (8.5 g, 93%) as a white solid. 1 H NMR (600MHz, DMSO-d6) δ4.49(s, 3H), 3.52(s, 6H), 1.37(s, 9H).
[0055]
[0056] A suspension of powdered KOH (2.3 g) and dry DMF (10 mL) was cooled to 0° C. under Ar protection. After compound 8 (1.5g) was dissolved in anhydrous THF (10mL), it was added dropwise to the reaction solution. After the addition was completed, it was reacted at 0°C for 10min, and 3.3mL of propyne bromide was added dropwise, and the temperature was raised to 35°C after 5min at 0°C. ℃ to react overnight, add deionized water (100 mL), CH 2 Cl 2 Extraction, washed with saturated brine, anhydrous Na 2 SO 4 It was dried, fi...
Embodiment 3
[0066]
[0067] Dissolve compound 6 (0.5g) and compound 14 (1.0g) in 10mL THF, Ar protection, add 10mL sodium ascorbate (0.115g) and CuSO 4 ·5H 2 O (0.07g) deionized aqueous solution, and react overnight at room temperature. Add deionized water, CH 2 Cl 2 Extraction, washed with saturated brine, anhydrous Na 2 SO 4 Drying, filtration, concentration, column separation (CH 2 Cl 2 :CH 3 OH = 40:1-20:1), to give 15 as a light red solid (1.14 g, 78%). 1 H NMR (600MHz, CDCl 3 )67.90 -7.83(m, 3H), 7.46(dd, J=8.9, 5.5Hz, 2H), 7.11(dd, J=5.2, 2.6Hz, 1H), 6.45(dt, J=8.9, 4.6Hz, 2H ), 6.39(d, J=2.4Hz, 2H), 6.30 -6.25(m, 2H), 5.91(d, J=9.2Hz, 2H), 5.51(td, J=9.4, 4.4Hz, 2H), 5.44 (t, J=9.1Hz, 2H), 5.38(d, J=3.3Hz, 2H), 5.15(dd, J=10.3, 8.0Hz, 2H), 5.00(dd, J=10.4, 3.4Hz, 2H) , 4.61(dd, J=12.5, 4.9Hz, 4H), 4.57(d, J=7.9Hz, 2H), 4.51(d, J=11.1Hz, 2H), 4.29(dd, J=10.5, 5.2Hz, 1H), 4.19-4.11(m, 6H), 4.04(td, J=9.4, 5.0Hz, 2H), 4.01-3.96(m, 2H), 3.93(t, J=6.7Hz, 2H), 3.65-3.58 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com