A kind of antiviral biological agent prepared by using lentinan and its production process
A technology for biological preparations and lentinan, applied in the directions of antiviral agents, microorganisms, microorganism-based methods, etc., can solve the problems of high requirements on reaction conditions, low antiviral activity, and unsatisfactory disease resistance effects, and achieves a simple process. , The effect of preventing viral diseases and treating avian viral diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 A process for preparing antiviral biological preparations using lentinan
[0029] Step 1 Bacillus subtilis activation
[0030] Take 1 tube of Bacillus subtilis lyophilized powder, add the lyophilized powder to 1ml YPD liquid medium prepared in advance, and shake to disperse;
[0031] The washed bacterial solution was streaked on the YPD solid medium, and placed in a 37°C incubator for 24 hours;
[0032] The components of the YPD liquid medium are as follows: glucose 20 g / L, yeast powder 10 g / L, peptone 20 g / L, and deionized water to make up the balance.
[0033] The components of the YPD solid medium are as follows: glucose 20 g / L, yeast powder 10 g / L, peptone 20 g / L, agar 20 g / L, and deionized water to make up the balance.
[0034] Step 2 seed liquid culture
[0035] Using lentinan to prepare a seed medium, and using the strain activated in step 1 to carry out fermentation and cultivation;
[0036] The components of the seed medium include: glucose 15g / L,...
Embodiment 2
[0060] Example 2 Carbon source single factor analysis experiment of fermentation medium
[0061] Adopt the method of Example 1 to prepare biological preparations, only change the carbon source of the fermentation medium in the fermentation step to carry out Examples 2-5; the carbon source of the fermentation medium used in Example 1 is 15g / L lentinan+5 g / L glucose
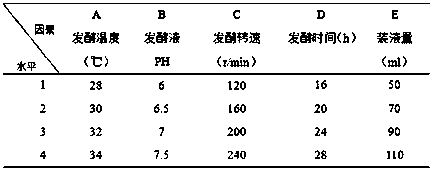
[0062] The carbon source of the fermentation medium that embodiment 2-5 adopts is shown in Table 2;
[0063] The carbon source of the fermentation medium that table 2 embodiment 2-5 adopts
[0064]
[0065] After testing, the antiviral activity of the biological preparations prepared in Examples 2-5 is shown in Table 3;
[0066] Table 3 The antiviral activity of the biological preparation prepared in Example 2-5
[0067]
[0068] It can be seen from Tables 2 and 3 that the carbon source of the fermentation medium has a great influence on the antiviral activity of the biological preparation, and the carbon ...
Embodiment 6
[0069] Nitrogen source single factor analysis experiment of embodiment 6 fermentation medium
[0070] Adopt the method of Example 1 to prepare biological preparations, only change the nitrogen source of the fermentation medium in the fermentation step to carry out Examples 6-10; the nitrogen source of the fermentation medium in Example 1 is: 12 g / L peptone+8 g / L urea;
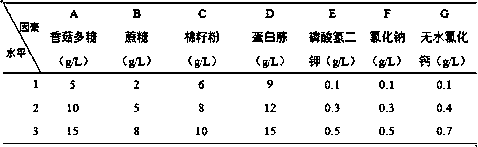
[0071] The nitrogen source of the fermentation medium that embodiment 6-10 adopts is shown in Table 4;
[0072] The nitrogen source of the fermentation medium that table 4 embodiment 6-10 adopts
[0073]
[0074] After testing, the antiviral activity of the biological preparations prepared in Examples 6-10 is shown in Table 5;
[0075] The antiviral activity of the biological preparation prepared in table 5 embodiment 6-10
[0076]
[0077] It can be known from Table 4 and 5 that the nitrogen source of the fermentation medium is preferably 12 g / L peptone+8 g / L cottonseed meal.
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com