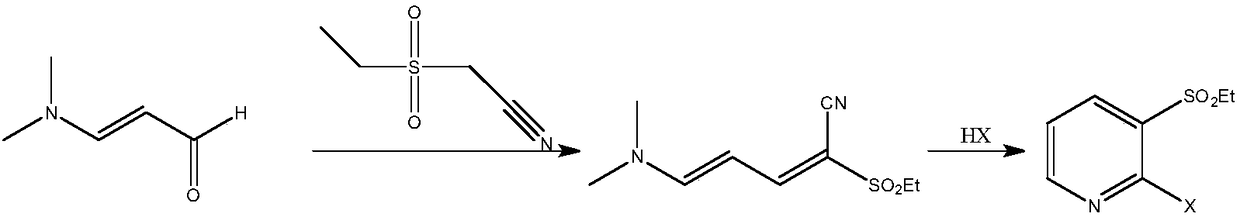
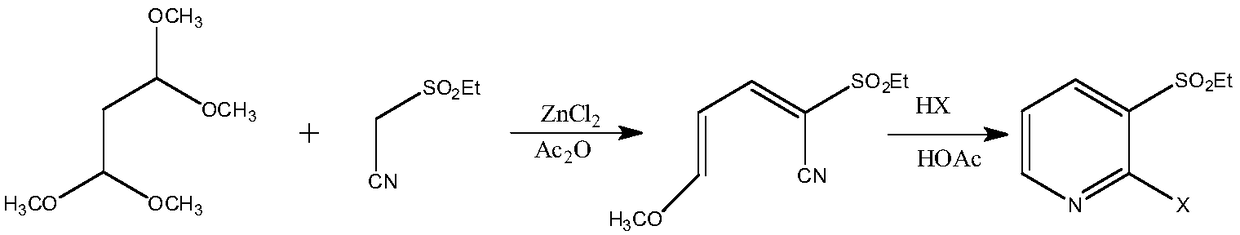
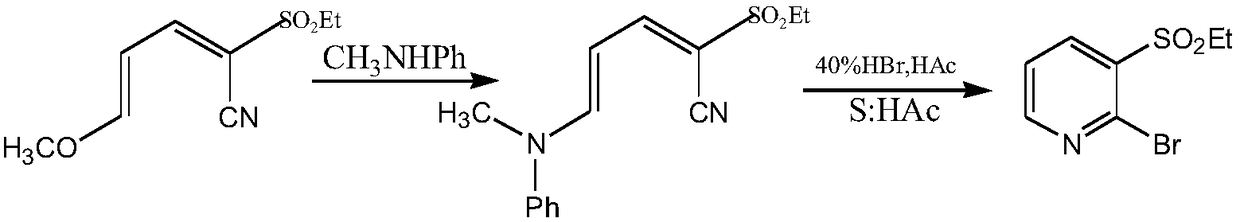
A method for synthesizing 2-halo-3-substituted hydrocarbylsulfonylpyridine and its intermediates by microwave method
A technology of hydrocarbylsulfonylpyridine and benzyl, which is applied in the field of microwave synthesis of 2-halo-3-substituted hydrocarbylsulfonylpyridine and its intermediates, which can solve the problems of low yield, difficult large-scale production, and many "three wastes", etc. problems, to achieve the effect of high product yield, less side reactions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: Preparation of 2-methylsulfonyl-5-(N,N-dimethyl)amino-2,4-pentadienenitrile
[0075] In a 500mL three-necked flask equipped with a thermometer, first add 59.6g (0.5mol) of methylcyanoethylsulfone and KHCO 3 5.0g, then add 62mL (0.5mol) of 3-dimethylaminoacrolein, mix well, put the prepared reactor into the microwave instrument, set the microwave radiation conditions, at a temperature of 60°C, a microwave power of 100W and a frequency of The reaction was performed under the condition of 915MHz. TLC detection (petroleum ether:dichloromethane 1:2 development, sublimation iodine color development) 3-dimethylaminoacrolein reacted completely, and the reaction time was 0.5h. Add sodium carbonate solution to adjust the pH=7-8, separate the layers, extract the aqueous layer with 100 mL of ethyl acetate x 3 times, combine the organic layers, dry overnight with molecular sieves, filter, and recover the solvent by distillation to obtain 97.3 g of a light yellow solid wit...
Embodiment 2
[0080] Example 2: Preparation of 2-ethylsulfonyl-5-(N,N-diethyl)amino-2,4-pentadienenitrile
[0081] In a 500mL three-necked flask equipped with a thermometer, first add 66.6g (0.5mol) of ethyl cyanoethylsulfone, 20mL of diethanolamine, and then add 124mL (1.0mol) of 3-diethylaminoacrolein, mix well, and prepare Put the reactor into a microwave instrument, set the microwave radiation conditions, and react under the conditions of 80°C temperature, 200W microwave power and 915MHz frequency, TLC detection (petroleum ether: methylene chloride 1:2, sublimation iodine Color development) The reaction of ethyl cyanoethyl sulfone is complete, and the reaction time is 0.5h. Add a 10% potassium hydroxide solution to adjust the pH to 7-8, separate the layers, extract the aqueous layer with 100 mL of propyl acetate x 3 times, combine the organic layers, evaporate the organic phase under reduced pressure to recover the propyl acetate, and obtain a shallow Yellow solid 115.7g, melting point...
Embodiment 3
[0082] Example 3: Preparation of 2-isopropylsulfonyl-5-(N,N-diethyl)amino-2,4-pentadienenitrile
[0083] In a 500mL three-necked flask equipped with a thermometer, first add 73mL (0.6mol) of isopropylcyanoethyl sulfone and 10mL of piperidine, then add 62mL (0.5mol) of 3-diethylaminoacrolein, mix well, and prepare Put the reactor into a microwave instrument, set the microwave radiation conditions, react under the conditions of temperature 120°C, microwave power 350W and frequency 2450MHz, TLC detection (petroleum ether: dichloromethane 1:2, sublimation iodine display Color) 3-diethylaminoacrolein reacted completely, the reaction time was 0.2h, adding potassium carbonate solution to adjust the pH=7-8, separating the layers, extracting the aqueous layer with 100mL of dichloromethane×3 times, combining the organic layers, adding anhydrous Na 2 SO 4 After drying and filtering, the organic phase was evaporated to dichloromethane and recovered to obtain 119.5 g of a light brown oil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com