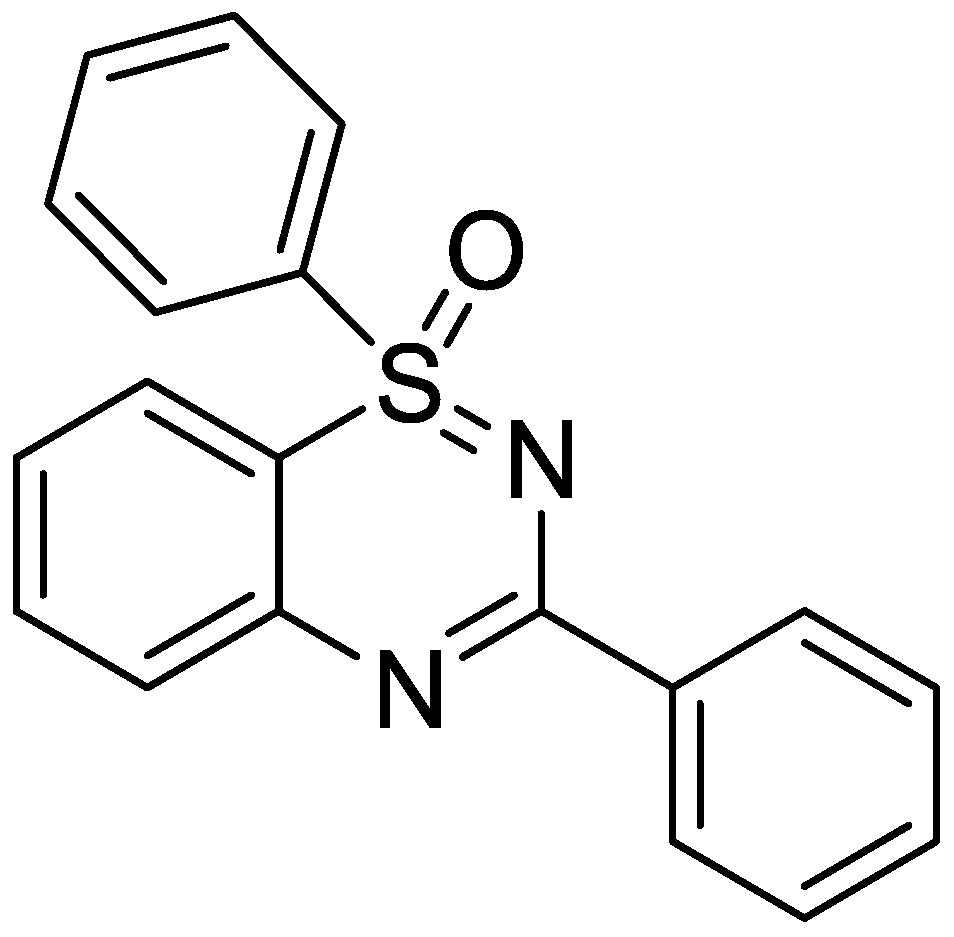
A kind of preparation method of 1,2,4-benzothiadiazine series compound
A technology of benzothiadiazines and compounds, which is applied in the field of preparation of 1,2,4-benzothiadiazines compounds, and achieves good application prospects, short reaction time and high chemoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023]
[0024] Dissolve the catalyst-carbonylcyclopentadiene cobalt iodide (9.2mg, 0.01mmol) silver bistrifluoromethanesulfonimide (16mg, 0.02mmol) in the mixed solvent tert-amyl alcohol / chloroform (1.0 mL, v:v=1:1), heated to 110°C in Biotage microwave reactor, then added dropwise reactant diphenylsulfinimide (43.4mg, 0.2mmol) and 3-phenyl-1, 4,2-Oxadiazol-5-one (64mg, 0.4mmol) and mixed solvent (1.0mL) were added to the reaction system, and the reaction was continued for 2 hours under air atmosphere, and the reaction was complete by TLC detection. During the post-treatment, the catalyst is removed by suction filtration through a sand core funnel equipped with silica gel, and the obtained filtrate is separated by flash column chromatography to obtain the pure product 1,3-diphenylbenzo[e][1,2,4]thiophene Oxadiazole 1-oxygen compound 3a. Yield: 85%. The following is the NMR experimental data of product 3a:
[0025] 1 H NMR (400MHz, CDCl 3 )δ:8.47-8.40(m,2H),7.88(dd,J=5...
Embodiment 2
[0028]
[0029] Dissolve the catalyst-carbonylcyclopentadiene cobalt iodide (230mg, 0.25mmol) silver bistrifluoromethanesulfonimide (400mg, 0.5mmol) in the mixed solvent tert-amyl alcohol / chloroform (25mL, v:v=1:1), heated to 110°C in Biotage microwave reactor, then added dropwise reactant diphenylsulfinimide (1.09g, 5.0mmol) and 3-phenyl-1,4, 2-Oxadiazol-5-one (1.6 g, 10 mmol) was added to the reaction system, and the reaction was continued for 3 hours under an air atmosphere, and the reaction was complete as detected by TLC. During the post-treatment, the catalyst is removed by suction filtration through a sand core funnel equipped with silica gel, and the obtained filtrate is separated by flash column chromatography to obtain the pure product 1,3-diphenylbenzo[e][1,2,4]thiophene Oxadiazole 1-oxygen compound 3a. Yield: 80%. The following is the NMR experimental data of product 3a:
[0030] 1 H NMR (400MHz, CDCl 3 )δ:8.47-8.40(m,2H),7.88(dd,J=5.4Hz,3.6Hz,2H),7.70-7.54...
Embodiment 3
[0033]
[0034] Dissolve the catalyst-carbonylcyclopentadiene cobalt iodide (230mg, 0.25mmol) silver bistrifluoromethanesulfonimide (400mg, 0.5mmol) in the mixed solvent tert-amyl alcohol / chloroform (25mL, v:v=1:1), heated to 120°C in an oil bath reactor, then added dropwise the reactant diphenylsulfinimide (1.09g, 5.0mmol) and 3-phenyl-1,4 , 2-oxadiazol-5-one (1.6 g, 10 mmol) was added to the reaction system, and the reaction was continued for 16 hours under air atmosphere, and the reaction was complete by TLC detection. During the post-treatment, the catalyst is removed by suction filtration through a sand core funnel equipped with silica gel, and the obtained filtrate is separated by flash column chromatography to obtain the pure product 1,3-diphenylbenzo[e][1,2,4]thiophene Oxadiazole 1-oxygen compound 3a. Yield: 69%. The following is the NMR experimental data of product 3a:
[0035] 1 H NMR (400MHz, CDCl 3 )δ:8.47-8.40(m,2H),7.88(dd,J=5.4Hz,3.6Hz,2H),7.70-7.54(m,5H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com