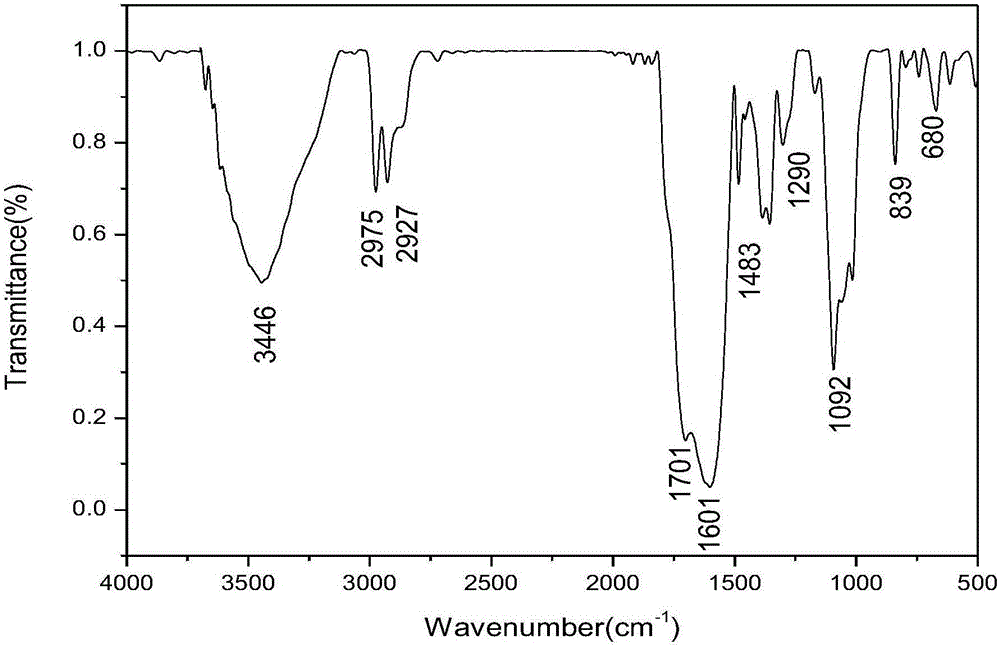
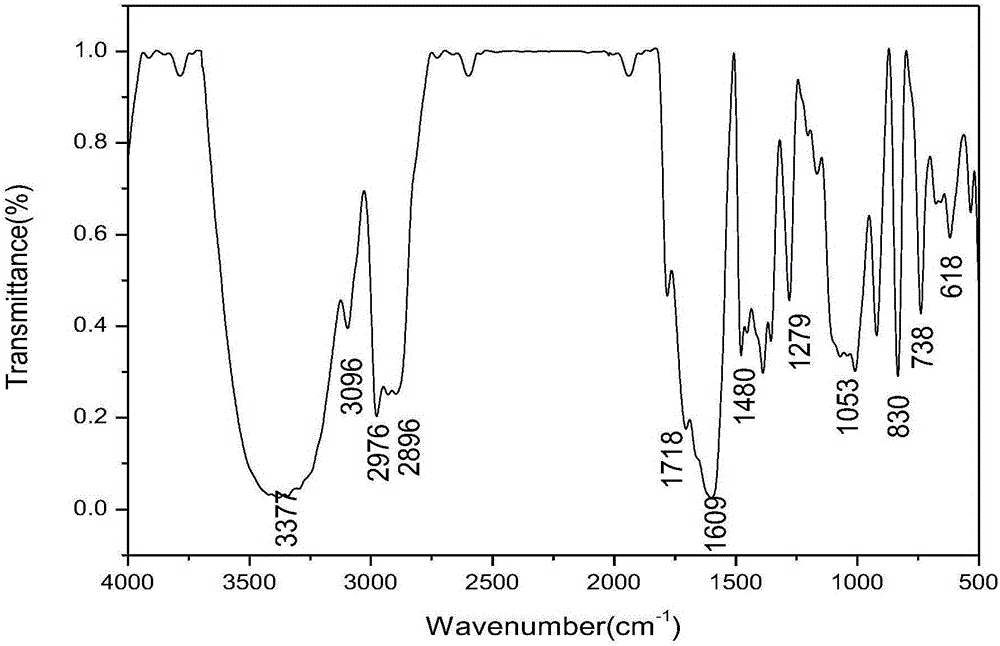
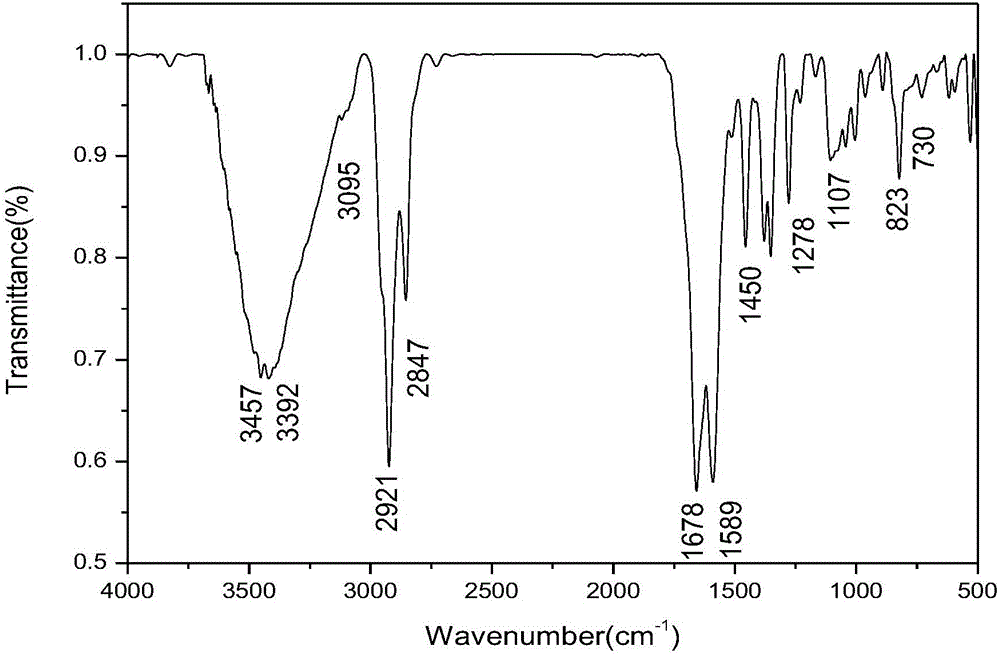
Ferrocenyl containing oxadiazolyl Mannich bases and preparation method thereof
A technology based on oxadiazolyl and amino oxadiazole is applied in the field of ferrocenyl-containing oxadiazolyl Mannich base and its preparation, and achieves the effects of high yield, mild reaction conditions and simple handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation method of aminooxadiazole containing substituents can refer to the literature: 2-amino-5-aryl-1,3,4-oxadiazole synthesis and process optimization, Liu Yuting, etc., "Journal of Shaanxi University of Science and Technology (Nature Science Edition), April 2015, Vol. 33, No. 2, pp. 79-82.
[0033] Concretely, the preparation method of aminooxadiazole containing substituent comprises the following steps:
[0034] 1) Add a mol semicarbazide hydrochloride and b mol absolute ethanol into a three-neck flask with a reflux condenser, and slowly add cmol aldehyde compounds dropwise, and reflux for 2 hours. After the reaction is completed, cool to room temperature, suction filter, and filter cake Dry to get hemiuret; wherein a:b:c=(0.9~1.2):(50~100):1; the structural formula of aldehydes is R 2 CHO;
[0035] 2) Add d mol hemiuret into a three-necked flask with a reflux condenser, and dropwise add e mol anhydrous sodium acetate dissolved in g mL glacial acetic acid...
Embodiment 1
[0037] Add 0.85mol 2-amino-5-(4-fluorophenyl)-1,3,4-oxadiazole to a dry three-necked flask with a reflux condenser, the mass percent concentration is 37% formaldehyde solution, 100mL of absolute ethanol as a solvent, during stirring, add 0.5mL of concentrated hydrochloric acid as a catalyst, then dropwise add a solution of acetylferrocene with a concentration of 0.5g / mL in absolute ethanol, heat and reflux at 80°C for 12h, pass through a TLC plate Monitor until complete reaction (developing solvent used by TLC is a mixture of dichloromethane and methanol with a volume ratio of 10:1). After the reaction, the reaction liquid was cooled, the solvent absolute ethanol was evaporated under reduced pressure, and then separated by silica gel column chromatography to obtain reddish-brown oily 3-(2-amino-5-p-fluorophenyl-1,3,4- Oxadiazole)-ethyl ferrocenyl ketone 126 mg, yield 63%. Wherein the formaldehyde solution contains 10mol of formaldehyde, and the absolute ethanol solution of ac...
Embodiment 2
[0040] Add 0.8mol 2-amino-5-(4-chlorophenyl)-1,3,4-oxadiazole to a dry three-necked flask with a reflux condenser, the mass percent concentration is 37% formaldehyde solution, 100mL of absolute ethanol as a solvent, during stirring, add 0.5mL of concentrated hydrochloric acid as a catalyst, then dropwise add a solution of acetylferrocene with a concentration of 0.5g / mL in absolute ethanol, heat and reflux at 80°C for 12h, pass through a TLC plate Monitor until complete reaction (developing solvent used by TLC is a mixture of dichloromethane and methanol with a volume ratio of 10:1). After the reaction, the reaction solution was cooled, the solvent absolute ethanol was evaporated under reduced pressure, and then separated by silica gel column chromatography to obtain reddish-brown oily 3-(2-amino-5-p-chlorophenyl-1,3,4- Oxadiazole)-ethyl ferrocenyl ketone, yield 65.7%. Wherein the formaldehyde solution contains 10mol of formaldehyde, and the absolute ethanol solution of acetyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com