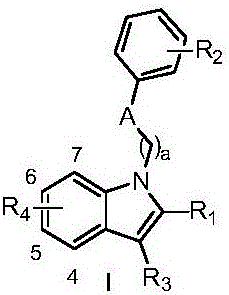
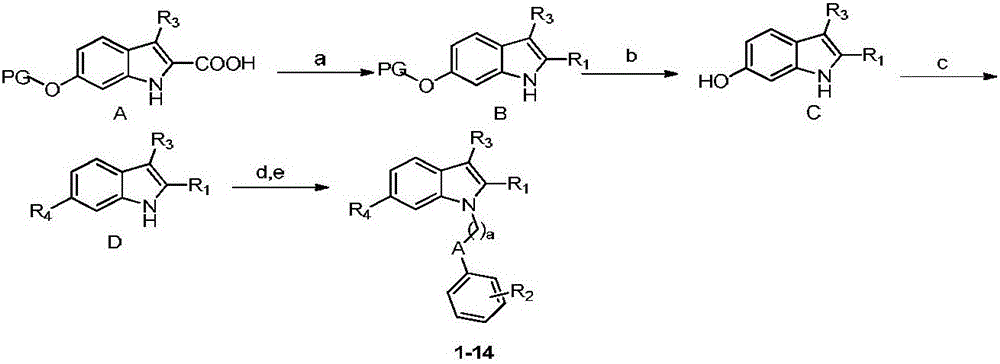
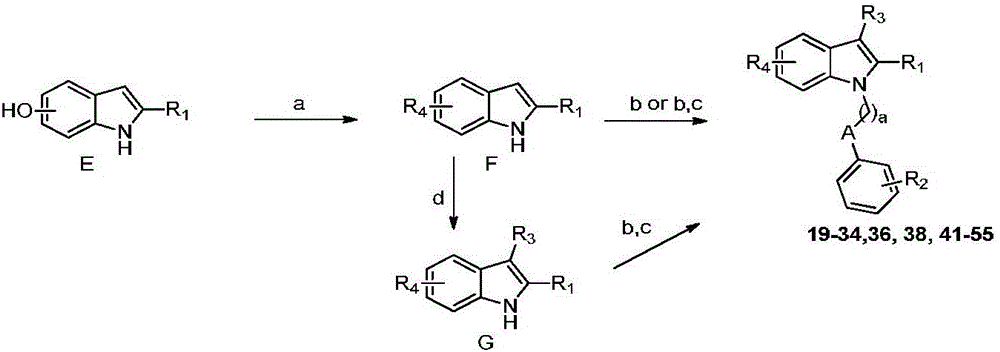
N-substituted indole derivative and application thereof
A technology of derivatives and indoles, applied in the field of preparing myeloid leukemia factor-1 and/or Bcl-2 protein inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] Example 1: 2-((2-carbamoyl-1-(2-phenoxyethyl)-1H-indol-6-yl)oxy)acetic acid
[0135] Compound 1A: 6-Methoxy-1H-indole-2-carboxamide
[0136] Add 6-methoxy-1H-indole-2-carboxylic acid (0.2g, 1.0mmol) and 30mL of anhydrous tetrahydrofuran to a 100mL eggplant-shaped flask, adjust the pH to 8 with DIEA, add HOBt (0.16g, 1.2 mmol), EDCI (0.23g, 1.2mmol) was reacted for 30min, 25% (w%) ammonia water (0.21g, 1.5mmol) was added, transferred to room temperature for 8h, and the reaction was stopped. Evaporate the solvent, add 100mL water and 100mL ethyl acetate for extraction, wash the organic layer with water (100mL×3), wash once with saturated aqueous sodium chloride solution, dry the organic layer with anhydrous sodium sulfate, filter with suction, and evaporate the filtrate to dryness to obtain light yellow Solid 6-methoxy-1H-indole-2-carboxamide (0.2 g), the crude product was directly carried on to the next reaction without isolation and purification. LC-MS: m / z=191.2[M+H]...
Embodiment 2
[0146] Example 2: 2-((2-carbamoyl-1-(2-(p-tolyloxy)ethyl)-1H-indol-6-yl)oxy)acetic acid
[0147] Compound 2A: ethyl 2-[(2-carbamoyl-1-(2-(p-tolyloxy)ethyl)-1H-indol-6-yl)oxy]acetate
[0148] Using compound 1C in Example 1 as a raw material, react with 1-(2-bromoethoxy)-4-methylbenzene according to the same reaction conditions to synthesize compound 2A, 2-[(2-carbamoyl-1- Ethyl (2-(p-tolyloxy)ethyl)-1H-indol-6-yl)oxy]acetate. Yield 56%. LC-MS: m / z=397.4[M+H] + .
[0149] Compound 2: 2-((2-carbamoyl-1-(2-(p-tolyloxy)ethyl)-1H-indol-6-yl)oxy)acetic acid
[0150] Using compound 2A as a raw material, the hydrolysis reaction was carried out under the same reaction conditions as compound 1 to obtain compound 2 as a white solid. Yield 78%, LC-MS m / z:367.0[M-H] - . 1 H-NMR (300MHz, DMSO-d6) δ: 7.94(d, J=10.5Hz, 1H), 7.54(d, J=8.7Hz, 1H), 7.28(s, 2H), 7.13(s, 1H), 7.03(d,J=8.3Hz,2H),6.85(dd,J=8.7,2.0Hz,1H),6.74(d,J=8.5Hz,2H),5.48(s,2H),4.91(t,J =5.2Hz, 2H), 4.23(t, J=5.4Hz, 2H)...
Embodiment 3
[0151] Example 3: 2-((2-carbamoyl-1-(2-(4-fluorophenoxy)ethyl)-1H-indol-6-yl)oxy)acetic acid
[0152] Compound 3A: Ethyl 2-[(2-carbamoyl-1-(2-(4-fluorophenoxy)ethyl)-1H-indol-6-yl)oxy]acetate
[0153] Using compound 1C in Example 1 as a raw material, react with 1-(2-bromoethoxy)-4-fluorobenzene according to the same reaction conditions to synthesize compound 3A, 2-[(2-carbamoyl-1-( 2-(4-Fluorophenoxy)ethyl)-1H-indol-6-yl)oxy]ethyl acetate. Yield 65%. LC-MS: m / z=401.4[M+H] + .
[0154] Compound 3: 2-((2-carbamoyl-1-(2-(4-fluorophenoxy)ethyl)-1H-indol-6-yl)oxy)acetic acid
[0155] Using compound 3A as a raw material, the hydrolysis reaction was carried out under the same reaction conditions as compound 1 to obtain compound 3 as a white solid. Yield 76%, LC-MS m / z:370.9[M-H] - . 1 H-NMR (300MHz, DMSO-d6) δ: 7.92(s, 1H), 7.54(d, J=8.7Hz, 1H), 7.32(d, J=8.5Hz, 2H), 7.13(s, 1H), 7.06(t, J=8.8Hz, 2H), 6.89(s, 1H), 6.87(s, 1H), 6.86-6.84(m, 1H), 5.51(s, 2H), 4.92(t, J=5.3Hz ,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com