Method for synthesis of chondroitin sulfate tetrasaccharide and intermediate compound
A kind of chondroitin sulfate, synthesis method technology, applied in the field of chemistry, can solve the problems of long route, low yield, many synthesis steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
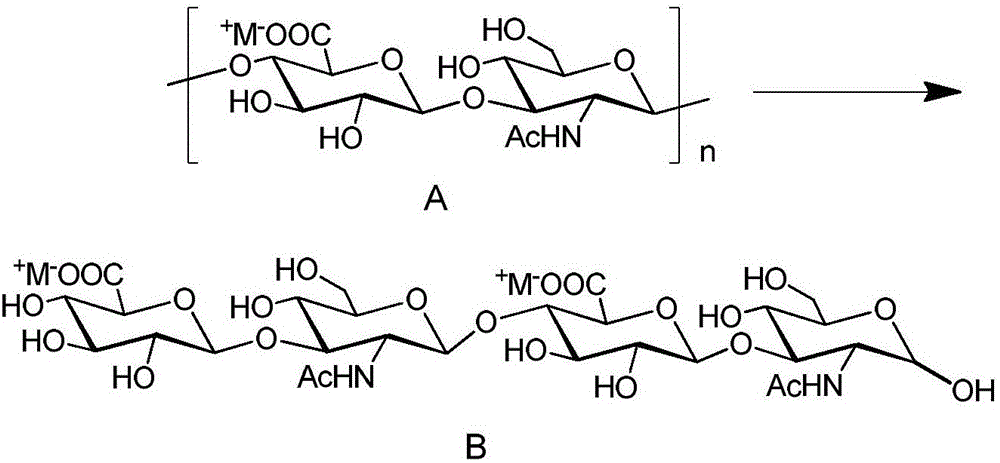
[0131]Example 1: (β-D-glucopyranosyl)-(1→3)-(2-deoxy-N-acetylamino-β-D-glucopyranosyl)-(1→4)-( Synthesis of β-D-glucopyranosyl)-(1→3)-2-deoxy-N-acetylamino-D-glucopyranose disodium salt
[0132] Take 51.0 g of hyaluronic acid dry powder (molecular weight of about 500,000) and place it in 2500 mL of 0.10 M sodium acetate-acetic acid buffer solution containing 0.15 M sodium chloride at pH = 5.00. Swelling (about 72h) is a colorless, uniform viscous colloidal liquid. The temperature of the jelly liquid was raised to 37.0° C. in a constant temperature water bath, and 1.0 g of hyaluronidase dry powder (purchased from Sigma-Aldrich Company, product number H-3506, Lot # SLBL1922V) was added at this time, and stirred at 37.0° C. for two weeks. After the reaction, the reaction solution was heated to boiling, and stirred at 105°C for 15 minutes. After the solution was no longer turbid, it was quickly cooled to room temperature, mixed with 1 / 20 volume of absolute ethanol, distilled unde...
Embodiment 2
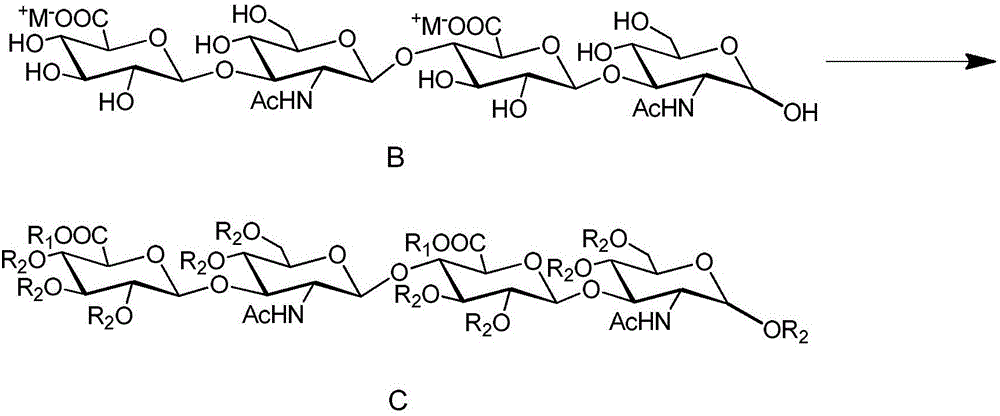
[0133] Example 2: (2,3,4-tri-O-acetyl-β-D-glucopyranosyl-methyl)-(1→3)-(4,6-di-O-acetyl- 2-Deoxy-N-acetylamino-β-D-glucopyranosyl)-(1→4)-(2,3-di-O-acetyl-β-D-glucopyranosyl) -Synthesis of -(1→3)-1,4,6-tri-O-acetyl-2-deoxy-N-acetylamino-D-glucopyranose
[0134] (β-D-glucopyranosyl)-(1→3)-(2-deoxy-N-acetylamino-β-D-glucopyranosyl)-(1→4)-(β-D -Glucopyranosyl)-(1→3)-2-deoxy-N-acetylamino-D-glucopyranose disodium salt crude product 96.0g was dissolved in 12L, 0.08M freshly refrigerated methanolic hydrogen chloride solution. Then the reaction solution was left standing at 4°C for 96h. After the reaction, the reaction solution was neutralized to neutral with triethylamine, then distilled under reduced pressure, and concentrated to dryness. Add 405 mL of pyridine, cool to 0° C., slowly dropwise add 230 mL of acetic anhydride, and slowly raise the reaction solution to room temperature, and stir at room temperature for 24 h. After the reaction was completed, the reaction liquid was ...
Embodiment 3
[0138] Example 3: (2,3,4-tri-O-acetyl-β-D-glucopyranosyl-methyl)-(1→3)-(4,6-di-O-acetyl- 2-Deoxy-N-acetylamino-β-D-glucopyranosyl)-(1→4)-(2,3-di-O-acetyl-β-D-glucopyranosyl) Synthesis of -(1→3)-4,6-di-O-acetyl-2-deoxy-N-acetylamino-D-glucopyranose
[0139] Take (2,3,4-tri-O-acetyl-β-D-glucuronomethyl)-(1→3)-(4,6-di-O-acetyl-2-deoxy-N -Acetylamino-β-D-glucopyranose)-(1→4)-(2,3-di-O-acetyl-β-D-glucuronyl)-(1→3)-1 , 30.2g (24.6mmol) of 4,6-tri-O-acetyl-2-deoxy-N-acetylamino-D-glucopyranose was dissolved in 240mL tetrahydrofuran, and 15.5mL (90.7mmol) was slowly added dropwise under stirring 3-N,N-dimethyl-aminopropylamine, and stirred at room temperature for 3h. Dilute the reaction solution with 650 mL of chloroform, wash the organic phase three times with 150 mL of 1M hydrochloric acid solution, extract the aqueous phase three times with 150 mL of chloroform, combine the organic phases, wash once with 230 mL saturated aqueous sodium bicarbonate solution, and wash once with sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com