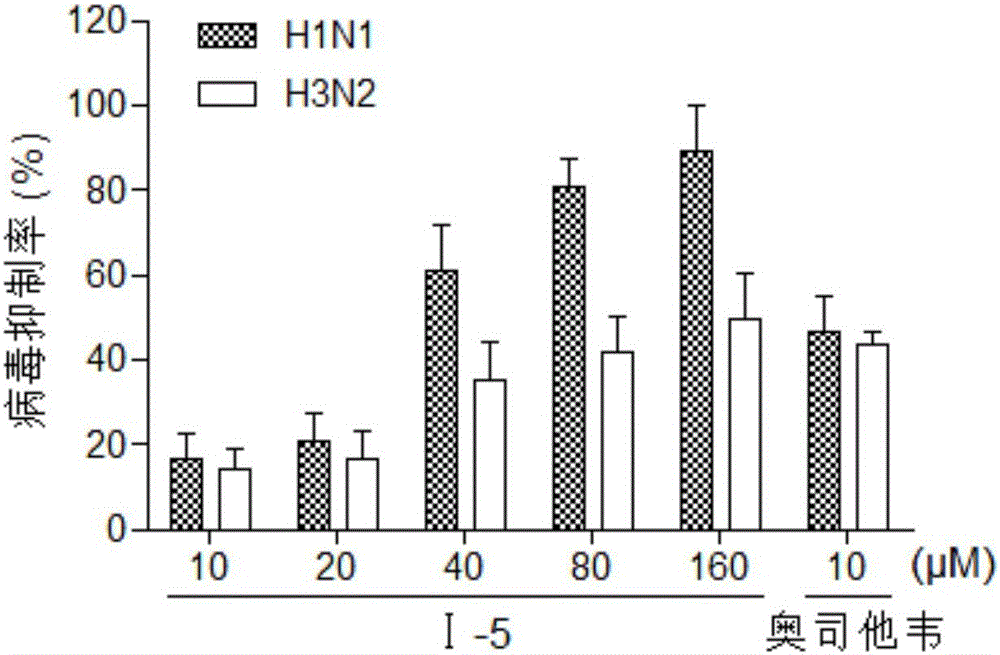
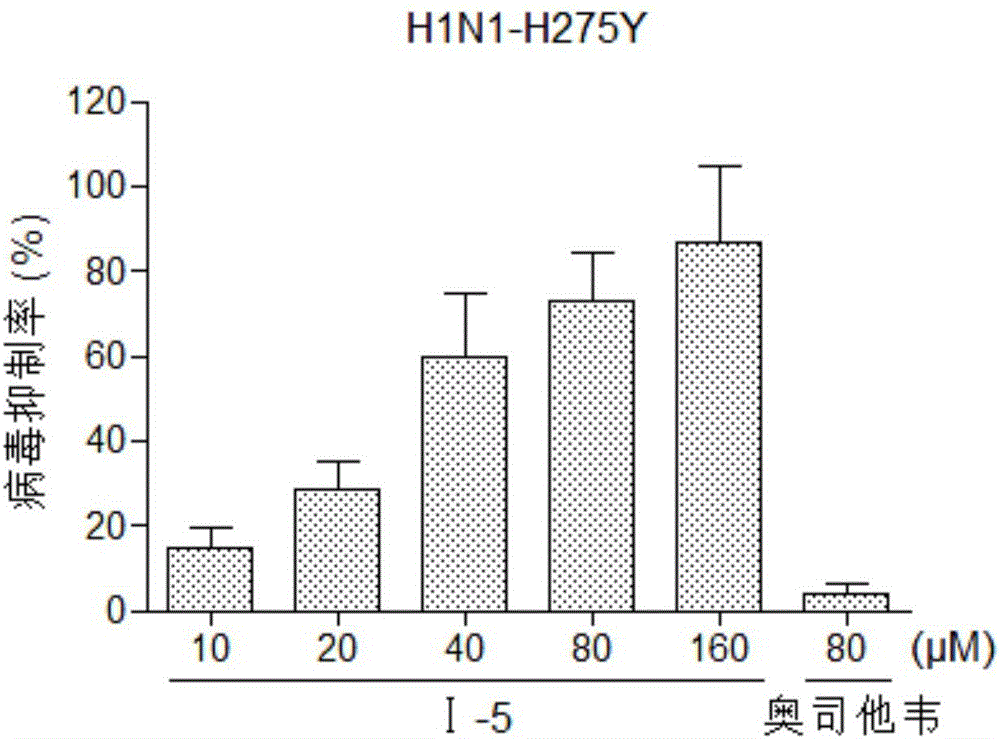
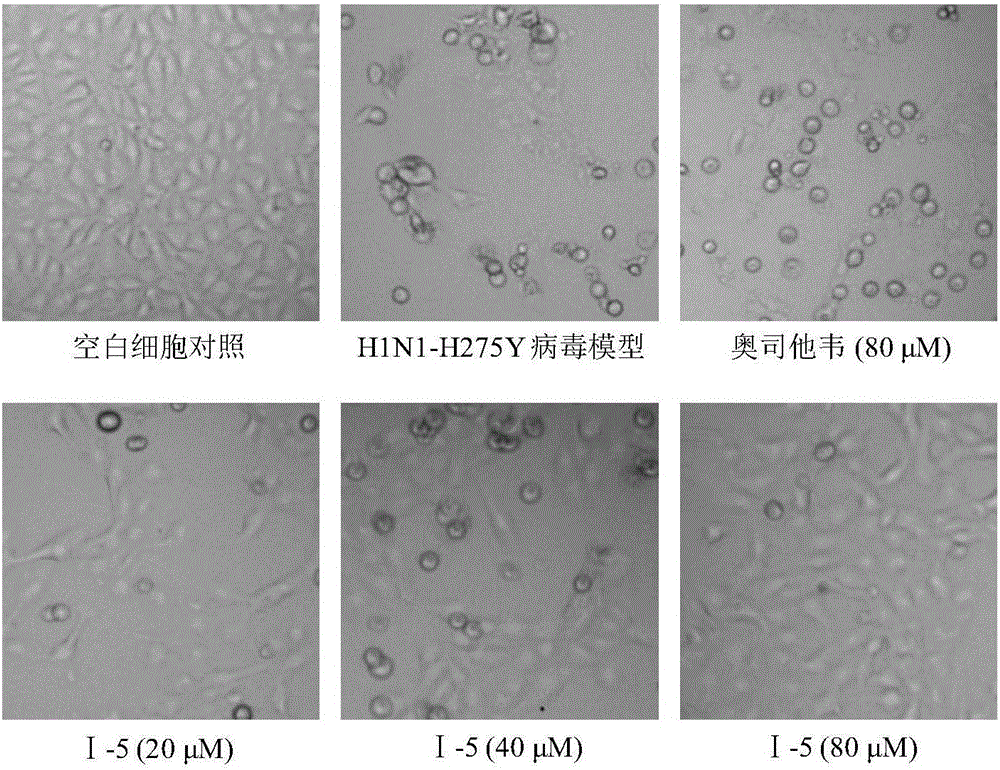
Benzoic acid triazole anti-influenza-virus compounds as well as preparation method and application thereof
A compound and drug technology, which is applied in the field of benzoic acid triazole anti-influenza virus compounds, can solve the problems of cumbersome steps, reduced affinity, high price, etc., and achieve the effect of broad application prospects, high-efficiency inhibition effect, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of key intermediate parts
[0057] Preparation of Diethyl 2-[4-(ethoxycarbonyl)anilino]malonate (C)
[0058] Add 30g (181.82mmol) ethyl p-aminobenzoate (A) and 20g (84.03mmol) diethyl bromomalonate (B) into a 500mL eggplant-shaped bottle, react at 115°C for 24h, dissolve and filter with ether, and the filtrate Wash 3 times with 10% hydrochloric acid, 3 times with water, and dry over anhydrous sodium sulfate. Filter and stand to precipitate crystals. Suction filtration and washing with a small amount of ether gave 23 g of colorless crystal C with a yield of 85.0%. m.p.: 68-72°C.
[0059] MS[M+H] + :324.1;
[0060] 1 H NMR (500MHz, CDCL 3 ), δ: 7.9(d, 2H, J=8.8Hz, ArH); 6.6(d, 2H, J=8.8Hz, ArH); 5.2(d, 1H, J=7.2Hz, CH); 4.8(d, 1H,J=7.2Hz,NH); 4.33~4.37(m,2H,CH 2 ); 4.3(m,4H,2CH 2 ); 1.35~1.38(t,3H,J=7.2Hz,CH 3 ); 1.11~1.14(t,6H,J=7.2Hz,2CH 3 ).
[0061] Preparation of 1-[4-(ethoxycarbonyl)phenyl]-5,5-dicarboxylate-2-pyrrolidone (D)
[0062] Add 5.6...
Embodiment 2
[0089] Synthesis of Ⅳ series intermediates
[0090] Ethyl 4-[2,2-(dimethylol)-5-oxopyrrolidinonyl]-3-[3-(2-methylbenzoyl)thioureido]benzoate (Ⅳ-1)
[0091] Add 0.41g (5.39mmol) of ammonium thiocyanate (5.39mmol) and 8mL of anhydrous acetone into a 25mL three-necked flask, drop 0.68g (4.40mmol) of o-toluyl chloride into 15mL of acetone within 5min, reflux for 20min, and then slowly drop Add 0.73 g (2.37 mmol) of ethyl 3-amino-4-[2,2-(dimethylol)-5-oxopyrrolidinonyl]benzoate (II) and dissolve it in 15 mL of acetone. Continue to reflux for 12h. The lysozyme was distilled off under reduced pressure, and the residue was separated by column chromatography (CH 2 CL:CH 3 OH=50:1), 0.41 g of white solid was obtained, and the yield was 35.44%. m.p.141-143°C.
[0092] [M+K] + :524.1;
[0093] 1 H NMR (300MHz, DMSO) δ (ppm): 12.55 (s, 1H, NH); 11.76 (s, 1H, NH); 9.08 (s, 1H, ArH); 7.85~7.88 (m, 1H, ArH); 7.41~7.54(m, 3H, ArH); 7.29~7.32(m, 2H, ArH); 5.31(s, 1H, OH); 4.89(s, 1H, O...
Embodiment 3
[0112] 4-[2,2-(Dihydroxymethyl)-5-oxopyrrolidinyl]-3-[5-(2-methylphenyl)-4H-1,2,4-triazolyl- Preparation of 3-amino)benzoic acid (Ⅰ-1)
[0113] Compound (Ⅳ-1) 0.25g (0.51mmol), 1 drop of acetic anhydride and 0.1ml of hydrazine hydrate were mixed in 10ml of absolute ethanol, N 2 Under protection, reflux for 6 hours, evaporate lysozyme under reduced pressure, and the residue is separated by column chromatography (CH 2 CL:CH 3 OH=50:1), 0.15 g of light yellow oil was obtained, yield 62.7%. Dissolve the oil in 10ml of methanol, add 1ml of 2N NaOH solution, stir at room temperature, monitor by TLC until the raw material point disappears, spin off the methanol, add 10% HCl to adjust the pH to 2-3, a white solid precipitates out, filter and dry to obtain White solid 40 mg, yield 29.0%. m.p.224~226℃
[0114] [M+H] + :438.2;
[0115] 1 H NMR (300MHz, DMSO) δ (ppm): 13.23 (s, 1H, COOH); 9.03 (s, 1H, NH); 8.91 (s, 1H, NH); 7.65~7.68 (m, 1H, ArH); 7.40~7.45(m, 1H, ArH); 7.28~7.34...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com